| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:09:43 UTC |
|---|
| Update Date | 2016-11-09 01:15:46 UTC |
|---|
| Accession Number | CHEM018331 |
|---|
| Identification |
|---|
| Common Name | Pivampicillin |
|---|
| Class | Small Molecule |
|---|
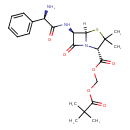
| Description | A penicillanic acid ester that is the pivaloyloxymethyl ester of ampicillin. It is a prodrug of ampicillin. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ampicillin pivaloyloxymethyl ester | ChEBI | | Pivaloyloxymethyl ampicillinate | ChEBI | | Pivampicilina | ChEBI | | Pivampicilline | ChEBI | | Pivampicillinum | ChEBI | | Pondocillin | Kegg | | Pivaloyloxymethyl ampicillinic acid | Generator | | Pivaloylampicillin | HMDB | | Berocillin | HMDB | | Serra pamies brand OF pivampicillin hydrochloride | HMDB | | Pivampicillin hydrochloride | HMDB | | Ampicillin pivaloyl ester | HMDB | | Ester, ampicillin pivaloyl | HMDB | | Hydrochloride, pivampicillin | HMDB | | Leo brand OF pivampicillin | HMDB | | Monohydrochloride, pivampicillin | HMDB | | Pivamiser | HMDB | | Pivampicillin monohydrochloride | HMDB |
|
|---|
| Chemical Formula | C22H29N3O6S |
|---|
| Average Molecular Mass | 463.547 g/mol |
|---|
| Monoisotopic Mass | 463.178 g/mol |
|---|
| CAS Registry Number | 33817-20-8 |
|---|
| IUPAC Name | [(2S,5R,6R)-6-[(2R)-2-amino-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbonyloxy]methyl 2,2-dimethylpropanoate |
|---|
| Traditional Name | pivampicillin |
|---|
| SMILES | [H][C@]12SC(C)(C)[C@@H](N1C(=O)[C@H]2NC(=O)[C@H](N)C1=CC=CC=C1)C(=O)OCOC(=O)C(C)(C)C |
|---|
| InChI Identifier | InChI=1S/C22H29N3O6S/c1-21(2,3)20(29)31-11-30-19(28)15-22(4,5)32-18-14(17(27)25(15)18)24-16(26)13(23)12-9-7-6-8-10-12/h6-10,13-15,18H,11,23H2,1-5H3,(H,24,26)/t13-,14-,15+,18-/m1/s1 |
|---|
| InChI Key | ZEMIJUDPLILVNQ-ZXFNITATSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenyl-1,2,4-triazoles. These are organic compounds containing a 1,2,4-triazole substituted by a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Triazoles |
|---|
| Direct Parent | Phenyl-1,2,4-triazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenyl-1,2,4-triazole
- Benzoic acid or derivatives
- Benzoic acid
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organic oxygen compound
- Organic nitrogen compound
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-4900000000-459356a4997f59da0598 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0pi0-1922100000-c66d44e9e103153e78e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-4921000000-e513d7e709a4cc359313 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9800000000-1d45b7913c48a4570128 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0290100000-8fa5f97dea1c97508c9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1691100000-3c1b28211f3589a2e3b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ke9-9630000000-8cb9ef389fad4dc01d14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-0322900000-3504b69e69554572682b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-7954200000-eb67af5b14cd5f26d30b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-5901000000-f16b60a416509c0adb29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01t9-1329700000-2f3c07f08b5464f78950 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udl-4934000000-ae133ff906d357093abb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9220000000-a556e0bfb78074f98c8d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01604 |
|---|
| HMDB ID | HMDB0015542 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Pivampicillin |
|---|
| Chemspider ID | 30899 |
|---|
| ChEBI ID | 8255 |
|---|
| PubChem Compound ID | 33478 |
|---|
| Kegg Compound ID | C11750 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|