| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:09:14 UTC |
|---|
| Update Date | 2016-11-09 01:15:46 UTC |
|---|
| Accession Number | CHEM018316 |
|---|
| Identification |
|---|
| Common Name | Benfotiamine |
|---|
| Class | Small Molecule |
|---|
| Description | A thioester that is a synthetic analogue of thiamine obtained by acylative cleavage of the thiazole ring and O-phospohorylation. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Benfotiamina | ChEBI | | Benfotiaminum | ChEBI | | Benphothiamine | ChEBI | | Benzoylthiamine monophosphate | ChEBI | | Benzoylthiamine O-monophosphate | ChEBI | | N-((4-Amino-2-methyl-5-pyrimidinyl)methyl)-N-(4-hydroxy-2-mercapto-1-methyl-1-butenyl)formamide S-benzoate O-phosphate | ChEBI | | S-Benzoylthiamine monophosphate | ChEBI | | S-Benzoylthiamine O-monophosphate | ChEBI | | Benzoylthiamine monophosphoric acid | Generator | | Benzoylthiamine O-monophosphoric acid | Generator | | N-((4-Amino-2-methyl-5-pyrimidinyl)methyl)-N-(4-hydroxy-2-mercapto-1-methyl-1-butenyl)formamide S-benzoic acid O-phosphoric acid | Generator | | S-Benzoylthiamine monophosphoric acid | Generator | | S-Benzoylthiamine O-monophosphoric acid | Generator | | BTMP-Benfo | MeSH | | Neurostop | MeSH | | Benfothiamine | MeSH | | Milgamma | MeSH |
|
|---|
| Chemical Formula | C19H23N4O6PS |
|---|
| Average Molecular Mass | 466.450 g/mol |
|---|
| Monoisotopic Mass | 466.108 g/mol |
|---|
| CAS Registry Number | 22457-89-2 |
|---|
| IUPAC Name | {[3-(benzoylsulfanyl)-4-{N-[(6-imino-2-methyl-1,6-dihydropyrimidin-5-yl)methyl]formamido}pent-3-en-1-yl]oxy}phosphonic acid |
|---|
| Traditional Name | [3-(benzoylsulfanyl)-4-{N-[(4-imino-2-methyl-3H-pyrimidin-5-yl)methyl]formamido}pent-3-en-1-yl]oxyphosphonic acid |
|---|
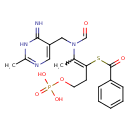
| SMILES | CC(N(CC1=CN=C(C)NC1=N)C=O)=C(CCOP(O)(O)=O)SC(=O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C19H23N4O6PS/c1-13(23(12-24)11-16-10-21-14(2)22-18(16)20)17(8-9-29-30(26,27)28)31-19(25)15-6-4-3-5-7-15/h3-7,10,12H,8-9,11H2,1-2H3,(H2,20,21,22)(H2,26,27,28) |
|---|
| InChI Key | BTNNPSLJPBRMLZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzoic acids and derivatives. These are organic compounds containing a carboxylic acid substituent attached to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Benzoic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzoic acid or derivatives
- Thiobenzoic acid or derivatives
- Benzoyl
- Aminopyrimidine
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Imidolactam
- Heteroaromatic compound
- Tertiary carboxylic acid amide
- Thiocarboxylic acid ester
- Amino acid or derivatives
- Carbothioic s-ester
- Carboxamide group
- Sulfenyl compound
- Azacycle
- Thiocarboxylic acid or derivatives
- Organoheterocyclic compound
- Carboxylic acid derivative
- Amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organosulfur compound
- Hydrocarbon derivative
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Primary amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052b-5902000000-eef1fb578efea4856082 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01b9-0819600000-f83de1cf3a67b419c198 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00xr-1926000000-669575172de9ec3034f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-2910000000-6297bd0e72aa5c82736e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03fr-7209300000-53c9b7a14421df34d35b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9011000000-baa459c55802adb04433 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-98917e5bfc9b005b0cfd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0100900000-9469dc9c77e37a9af145 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00xr-2925500000-d4a96d31948bf5f7d202 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00e9-4922000000-e8ea330c6095a3ce5c65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004j-9010100000-c7f77ffa9289fae934c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-15030017c51fd2fb76a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-2c0e23e32e082d7e1719 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11748 |
|---|
| HMDB ID | HMDB0248951 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Benfotiamine |
|---|
| Chemspider ID | 2230 |
|---|
| ChEBI ID | 41039 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|