| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:08:59 UTC |
|---|
| Update Date | 2016-11-09 01:15:46 UTC |
|---|
| Accession Number | CHEM018312 |
|---|
| Identification |
|---|
| Common Name | Dilazep |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
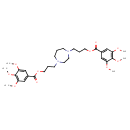
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Asta C 4898 | MeSH | | Trimethoxybenzoate, biopropazepan | MeSH | | C 4898, Asta | MeSH | | Biopropazepan trimethoxybenzoate | MeSH | | Cormelian | MeSH | | Dilazep dihydrochloride | ChEMBL | | 3-[4-[3-(3,4,5-Trimethoxybenzoyl)oxypropyl]-1,4-diazepan-1-yl]propyl 3,4,5-trimethoxybenzoic acid | Generator | | 4898, Asta C | MeSH | | Dilazep | MeSH |
|
|---|
| Chemical Formula | C31H44N2O10 |
|---|
| Average Molecular Mass | 604.697 g/mol |
|---|
| Monoisotopic Mass | 604.300 g/mol |
|---|
| CAS Registry Number | 35898-87-4 |
|---|
| IUPAC Name | 3-{4-[3-(3,4,5-trimethoxybenzoyloxy)propyl]-1,4-diazepan-1-yl}propyl 3,4,5-trimethoxybenzoate |
|---|
| Traditional Name | dilazep |
|---|
| SMILES | COC1=CC(=CC(OC)=C1OC)C(=O)OCCCN1CCCN(CCCOC(=O)C2=CC(OC)=C(OC)C(OC)=C2)CC1 |
|---|
| InChI Identifier | InChI=1S/C31H44N2O10/c1-36-24-18-22(19-25(37-2)28(24)40-5)30(34)42-16-8-12-32-10-7-11-33(15-14-32)13-9-17-43-31(35)23-20-26(38-3)29(41-6)27(21-23)39-4/h18-21H,7-17H2,1-6H3 |
|---|
| InChI Key | QVZCXCJXTMIDME-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gallic acid and derivatives. Gallic acid and derivatives are compounds containing a 3,4,5-trihydroxybenzoic acid moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Gallic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gallic acid or derivatives
- M-methoxybenzoic acid or derivatives
- P-methoxybenzoic acid or derivatives
- Benzoate ester
- Anisole
- Benzoyl
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- 1,4-diazepane
- Alkyl aryl ether
- Diazepane
- Dicarboxylic acid or derivatives
- Tertiary aliphatic amine
- Tertiary amine
- Amino acid or derivatives
- Carboxylic acid ester
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Ether
- Organic oxide
- Amine
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1013019000-91659d3509c889d899a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6x-2549011000-471a4eecc17ed21d463b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6x-6975040000-9fa5cd810dd9a4fe0f73 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0121009000-aeeb2d176c3780e57569 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0694042000-23e288c04f1a1747b703 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-1492030000-14ffaa94a96a4a6cb735 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0200009000-f2989381ad46e83b51a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0603459000-01240ff07e5d46cc8199 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-0912000000-a8998b7c34a1f2e8ccf8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0020009000-d286b4fbb083ffd95900 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zmu-0010091000-1fabe67e6a31f62aa585 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gb9-2940242000-dc25980d7df51d4a70a2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13715 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Dilazep |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 3074 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|