| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:08:57 UTC |
|---|
| Update Date | 2016-11-09 01:15:46 UTC |
|---|
| Accession Number | CHEM018311 |
|---|
| Identification |
|---|
| Common Name | Piromidic acid |
|---|
| Class | Small Molecule |
|---|
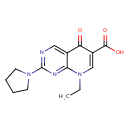
| Description | A pyridopyrimidine that is 5-oxo-5,8-dihydropyrido[2,3-d]pyrimidine-6-carboxylic acid, substituted at position 2 by a pyrrolidin-1-yl group and at position 8 by an ethyl group. A synthetic antibacterial which is used for the treatment of urinary tract and intestinal infections. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 8-Ethyl-5,8-dihydro-5-oxo-2-(1-pyrrolidinyl)pyrido(2,3-D)pyrimidine-6-carboxylic acid | ChEBI | | Acide piromidique | ChEBI | | Acido piromidico | ChEBI | | Acidum piromidicum | ChEBI | | Actrun C | ChEBI | | Bactramyl | ChEBI | | Enterol | ChEBI | | Gastrurol | ChEBI | | Panacid | ChEBI | | Pyromidic acid | ChEBI | | Reelon | ChEBI | | Septural | ChEBI | | Uropir | ChEBI | | 8-Ethyl-5,8-dihydro-5-oxo-2-(1-pyrrolidinyl)pyrido(2,3-D)pyrimidine-6-carboxylate | Generator | | Pyromidate | Generator | | Piromidate | Generator | | PA | KEGG | | Acid, piromidic | MeSH |
|
|---|
| Chemical Formula | C14H16N4O3 |
|---|
| Average Molecular Mass | 288.307 g/mol |
|---|
| Monoisotopic Mass | 288.122 g/mol |
|---|
| CAS Registry Number | 19562-30-2 |
|---|
| IUPAC Name | 8-ethyl-5-oxo-2-(pyrrolidin-1-yl)-5H,8H-pyrido[2,3-d]pyrimidine-6-carboxylic acid |
|---|
| Traditional Name | enterol |
|---|
| SMILES | CCN1C=C(C(O)=O)C(=O)C2=CN=C(N=C12)N1CCCC1 |
|---|
| InChI Identifier | InChI=1S/C14H16N4O3/c1-2-17-8-10(13(20)21)11(19)9-7-15-14(16-12(9)17)18-5-3-4-6-18/h7-8H,2-6H2,1H3,(H,20,21) |
|---|
| InChI Key | RCIMBBZXSXFZBV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrido[2,3-d]pyrimidines. Pyrido[2,3-d]pyrimidines are compounds containing the pyrido[2,3-d]pyrimidine ring system, which is a pyridopyrimidine isomer with three ring nitrogen atoms at the 1-, 3-, and 8- position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridopyrimidines |
|---|
| Sub Class | Pyrido[2,3-d]pyrimidines |
|---|
| Direct Parent | Pyrido[2,3-d]pyrimidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrido[2,3-d]pyrimidine
- Pyridine carboxylic acid
- Pyridine carboxylic acid or derivatives
- Dialkylarylamine
- Aminopyrimidine
- Pyrimidine
- Pyridine
- Heteroaromatic compound
- Vinylogous amide
- Pyrrolidine
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-006x-0980000000-e0572e12c19b3c4fdc72 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-6dac86f0218e62a678da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dv-0090000000-1c0f05e8b35af7223253 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006x-6790000000-78a7c8439d721f499128 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-0090000000-dc4762fae5c89796d99a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014l-0090000000-69c6b20abe5c1bb4e3e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02ta-4490000000-8348d7b2352fe3679cbf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13744 |
|---|
| HMDB ID | HMDB0256609 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Piromidic_acid |
|---|
| Chemspider ID | 4689 |
|---|
| ChEBI ID | 32019 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|