| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:08:55 UTC |
|---|
| Update Date | 2016-11-09 01:15:46 UTC |
|---|
| Accession Number | CHEM018309 |
|---|
| Identification |
|---|
| Common Name | Ornidazole |
|---|
| Class | Small Molecule |
|---|
| Description | Ornidazole has been used in trials studying the prevention of Elective Colorectal Surgery. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Tiberal | MeSH | | 1-(2-Hydroxy-3-chloropropyl)-2-methyl-5-nitroimidazole | ChEBI | | 1-(3-chloro-2-Hydroxypropyl)-2-methyl-5-nitroimidazole | ChEBI | | alpha-(Chlormethyl)-2-methyl-5-nitro-imidazol-1-aethanol | ChEBI | | alpha-(Chloromethyl)-2-methyl-5-nitro-1H-imidazole-1-ethanol | ChEBI | | Ornidazol | ChEBI | | Ornidazolum | ChEBI | | ro 7-0207 | ChEBI | | a-(Chlormethyl)-2-methyl-5-nitro-imidazol-1-aethanol | Generator | | α-(chlormethyl)-2-methyl-5-nitro-imidazol-1-aethanol | Generator | | a-(Chloromethyl)-2-methyl-5-nitro-1H-imidazole-1-ethanol | Generator | | α-(chloromethyl)-2-methyl-5-nitro-1H-imidazole-1-ethanol | Generator | | Ornidazole | MeSH |
|
|---|
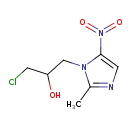
| Chemical Formula | C7H10ClN3O3 |
|---|
| Average Molecular Mass | 219.630 g/mol |
|---|
| Monoisotopic Mass | 219.041 g/mol |
|---|
| CAS Registry Number | 16773-42-5 |
|---|
| IUPAC Name | 1-chloro-3-(2-methyl-5-nitro-1H-imidazol-1-yl)propan-2-ol |
|---|
| Traditional Name | madelen |
|---|
| SMILES | CC1=NC=C(N1CC(O)CCl)N(=O)=O |
|---|
| InChI Identifier | InChI=1S/C7H10ClN3O3/c1-5-9-3-7(11(13)14)10(5)4-6(12)2-8/h3,6,12H,2,4H2,1H3 |
|---|
| InChI Key | IPWKIXLWTCNBKN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as nitroimidazoles. Nitroimidazoles are compounds containing an imidazole ring which bears a nitro group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Imidazoles |
|---|
| Direct Parent | Nitroimidazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2,5-trisubstituted-imidazole
- Nitroaromatic compound
- Nitroimidazole
- Trisubstituted imidazole
- N-substituted imidazole
- Heteroaromatic compound
- Chlorohydrin
- Halohydrin
- C-nitro compound
- Secondary alcohol
- Organic nitro compound
- Allyl-type 1,3-dipolar organic compound
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organic oxoazanium
- Hydrocarbon derivative
- Organonitrogen compound
- Organochloride
- Organooxygen compound
- Organohalogen compound
- Organopnictogen compound
- Alkyl chloride
- Organic oxygen compound
- Alcohol
- Organic nitrogen compound
- Alkyl halide
- Organic oxide
- Organic zwitterion
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-004i-3911000000-5b4872e026a447ba42bd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00fr-0590000000-51e17e00bdee7b7e70f6 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00fr-1590000000-10d4a11d06fa5ff22012 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-004i-3911000000-5b4872e026a447ba42bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-fe43bab832f5c5f00d08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0940000000-bf0110df83d86673d2ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05po-7900000000-2d1c03324b8c7cfc3e4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-7a2c1637dab6872fd465 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01dl-9740000000-10d11e586f5ff12cabcc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-4b5936f742c4bd5ffab2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13026 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ornidazole |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 28061 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|