| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:08:44 UTC |
|---|
| Update Date | 2016-11-09 01:15:46 UTC |
|---|
| Accession Number | CHEM018305 |
|---|
| Identification |
|---|
| Common Name | Alcuronium chloride |
|---|
| Class | Small Molecule |
|---|
| Description | Chloride salt of alcuronium. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
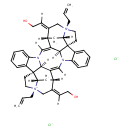
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4,4'-Didemethyl-4,4'-di-2-propenyltoxiferine I dichloride | ChEBI | | Alcuronii chloridum | ChEBI | | Alcuronium dichloride | ChEBI | | Alloferin | ChEBI | | Chlorure d'alcuronium | ChEBI | | Cloruro de alcuronio | ChEBI | | Diallylnortoxiferine dichloride | ChEBI | | N,N'-diallylnortoxiferinium dichloride | ChEBI | | Alcuronium | MeSH | | Allnortoxiferine | MeSH | | Dialferine | MeSH | | Diallylnortoxiferine | MeSH | | Dichloride, alcuronium | MeSH | | Dichloride, N,n'-diallylnortoxiferinium | MeSH | | Alcuronium chloride | MeSH |
|
|---|
| Chemical Formula | C44H50Cl2N4O2 |
|---|
| Average Molecular Mass | 737.810 g/mol |
|---|
| Monoisotopic Mass | 736.331 g/mol |
|---|
| CAS Registry Number | 15180-03-7 |
|---|
| IUPAC Name | (1S,11S,13S,14R,17S,27S,28E,30R,33S,35S,37E,38S)-28,37-bis(2-hydroxyethylidene)-14,30-bis(prop-2-en-1-yl)-8,14,24,30-tetraazaundecacyclo[25.5.2.2¹¹,¹⁴.1¹,⁸.1¹⁰,¹⁷.0²,⁷.0¹³,¹⁷.0¹⁸,²³.0³⁰,³³.0²⁴,³⁵.0²⁶,³⁸]octatriaconta-2,4,6,9,18,20,22,25-octaene-14,30-diium dichloride |
|---|
| Traditional Name | alcuronium dichloride |
|---|
| SMILES | [Cl-].[Cl-].[H]\C(CO)=C1/C[N@+]2(CC=C)CC[C@]34C5=CC=CC=C5N5\C([H])=C6/[C@]7([H])N(\C([H])=C(/[C@@]35[H])[C@@]1([H])C[C@]24[H])C1=CC=CC=C1[C@@]71CC[N@@+]2(CC=C)C\C(=C(/[H])CO)[C@]6([H])C[C@@]12[H] |
|---|
| InChI Identifier | InChI=1S/C44H50N4O2.2ClH/c1-3-17-47-19-15-43-35-9-5-7-11-37(35)45-26-34-32-24-40-44(16-20-48(40,18-4-2)28-30(32)14-22-50)36-10-6-8-12-38(36)46(42(34)44)25-33(41(43)45)31(23-39(43)47)29(27-47)13-21-49;;/h3-14,25-26,31-32,39-42,49-50H,1-2,15-24,27-28H2;2*1H/q+2;;/p-2/b29-13-,30-14-,33-25-,34-26-;;/t31-,32-,39-,40-,41-,42-,43+,44+,47-,48-;;/m0../s1 |
|---|
| InChI Key | CPYGBGOXCJJJGC-GKLGUMFISA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as strychnos alkaloids. These are alkaloids having a core structure based on the strychnan, stemmadenine (seco-curan), or the akuammicine (curan) skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Strychnos alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Strychnos alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Strychnan skeleton
- Akuammicine-skeleton
- Stemmadenine-skeleton
- Curan skeleton
- Carbazole
- Indolizidine
- Indole or derivatives
- Tertiary aliphatic/aromatic amine
- Aralkylamine
- Benzenoid
- N-alkylpyrrolidine
- Piperidine
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Pyrrolidine
- Tertiary amine
- Allylamine
- Azacycle
- Organoheterocyclic compound
- Enamine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic chloride salt
- Organic salt
- Organic zwitterion
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-015i-1000009800-610d590205a8d1f52217 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01c3-4000009300-7029bed58c7e8215e938 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-8003009000-e6fc96a76d3bc22dfff6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000900-54ed7ced1e8935e6ba30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1000009800-745430b523c675152b4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fb9-3004109100-07fc93cdc16a9befca9e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Alcuronium chloride |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 31185 |
|---|
| PubChem Compound ID | 21158559 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|