| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:02:20 UTC |
|---|
| Update Date | 2016-11-09 01:15:44 UTC |
|---|
| Accession Number | CHEM018163 |
|---|
| Identification |
|---|
| Common Name | Egtazic Acid |
|---|
| Class | Small Molecule |
|---|
| Description | A diether that is ethylene glycol in which the hydrogens of the hydroxy groups have been replaced by 2-ethyl group respectively. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
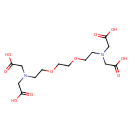
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,12-Bis(carboxymethyl)-6,9-dioxa-3,12-diazatetradecanedioic acid | ChEBI | | [Ethylenebis(oxyethylenenitrilo)]tetraacetic acid | ChEBI | | EGTA | ChEBI | | Egtazic acid | ChEBI | | Ethylene glycol bis(beta-aminoethyl ether)-N,N,n',n'-tetraacetic acid | ChEBI | | Ethylene glycol-O,o'-bis(2-aminoethyl)-N,N,n',n'-tetraacetic acid | ChEBI | | H4EGta | ChEBI | | 3,12-Bis(carboxymethyl)-6,9-dioxa-3,12-diazatetradecanedioate | Generator | | [Ethylenebis(oxyethylenenitrilo)]tetraacetate | Generator | | Egtazate | Generator | | Ethylene glycol bis(b-aminoethyl ether)-N,N,n',n'-tetraacetate | Generator | | Ethylene glycol bis(b-aminoethyl ether)-N,N,n',n'-tetraacetic acid | Generator | | Ethylene glycol bis(beta-aminoethyl ether)-N,N,n',n'-tetraacetate | Generator | | Ethylene glycol bis(β-aminoethyl ether)-N,N,n',n'-tetraacetate | Generator | | Ethylene glycol bis(β-aminoethyl ether)-N,N,n',n'-tetraacetic acid | Generator | | Ethylene glycol-O,o'-bis(2-aminoethyl)-N,N,n',n'-tetraacetate | Generator | | Ethylene glycol bis(2-aminoethyl)tetraacetate | Generator | | 2-[2-[2-[2-[Bis(carboxymethyl)amino]ethoxy]ethoxy]ethyl-(carboxymethyl)amino]acetate | Generator | | Acid, egtazic | MeSH | | Magnesium egta | MeSH | | GEDTA | MeSH | | Egtazic acid sodium salt | MeSH | | Ethylene glycol bis(2-aminoethyl ether)tetraacetic acid | MeSH | | Magnesium-egta | MeSH | | Tetrasodium egta | MeSH | | Egtazic acid disodium salt | MeSH | | EGATA | MeSH | | Glycoletherdiamine-N,N,n',n'-tetraacetic acid | MeSH | | EGTA, tetrasodium | MeSH | | Egtazic acid potassium salt | MeSH | | Ethylene glycol tetraacetic acid | MeSH | | Ethylenebis(oxyethylenenitrile)tetraacetic acid | MeSH |
|
|---|
| Chemical Formula | C14H24N2O10 |
|---|
| Average Molecular Mass | 380.350 g/mol |
|---|
| Monoisotopic Mass | 380.143 g/mol |
|---|
| CAS Registry Number | 67-42-5 |
|---|
| IUPAC Name | 3,12-bis(carboxymethyl)-6,9-dioxa-3,12-diazatetradecanedioic acid |
|---|
| Traditional Name | EGTA |
|---|
| SMILES | OC(=O)CN(CCOCCOCCN(CC(O)=O)CC(O)=O)CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C14H24N2O10/c17-11(18)7-15(8-12(19)20)1-3-25-5-6-26-4-2-16(9-13(21)22)10-14(23)24/h1-10H2,(H,17,18)(H,19,20)(H,21,22)(H,23,24) |
|---|
| InChI Key | DEFVIWRASFVYLL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetracarboxylic acids and derivatives. These are carboxylic acids containing exactly four carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Tetracarboxylic acids and derivatives |
|---|
| Direct Parent | Tetracarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetracarboxylic acid or derivatives
- Alpha-amino acid
- Alpha-amino acid or derivatives
- Amino acid or derivatives
- Amino acid
- Tertiary aliphatic amine
- Tertiary amine
- Carboxylic acid
- Dialkyl ether
- Ether
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-1921000000-44692988d23e1f23c4c4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_4_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001r-0219000000-8711a231ba057a7401a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1859000000-568fff87b59a970e443e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01p9-1950000000-1707e60a92b90d9b7349 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0219000000-4e1dc25641ef7061ca8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0839000000-c5c0082a2e0766928840 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-5910000000-d81f3e2399de70e37606 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0009000000-cd2d69b44b22e24edb7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0019000000-7703087f6187ae233960 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03k9-7911000000-bee65bc984e43fd5c9ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0400-0009000000-03f8da3ac9c316324d10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00n0-0679000000-425f021afac1d1f14309 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0c00-6920000000-b38f158d015fafa9e17b | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | EGTA_(chemical) |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 30740 |
|---|
| PubChem Compound ID | 6207 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|