| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:01:54 UTC |
|---|
| Update Date | 2016-11-09 01:15:44 UTC |
|---|
| Accession Number | CHEM018148 |
|---|
| Identification |
|---|
| Common Name | Docebenone |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of benzoquinones that is p-benzoquinone in which the hydrogens are substituted by three methyl groups and a 12-hydroxydodeca-5,10-diyn-1-yl group. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
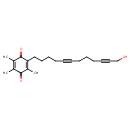
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3,5-Trimethyl-6-(12-hydroxy-5,10-dodecadiynyl)-1,4-benzoquinone | ChEBI | | 2-(12-Hydroxy-5,10-dodecadiynyl)-3,5,6-trimethyl-p-benzoquinone | ChEBI | | 2-(12-Hydroxydodeca-5,10-diynyl)-3,5,6-trimethyl-1,4-benzoquinone | ChEBI | | 6-(12-Hydroxydodeca-5,10-diyn-1-yl)-2,3,5-trimethyl-1,4-benzoquinone | ChEBI | | AA-861 | ChEBI | | AA861 | ChEBI | | Docebenona | ChEBI | | Docebenonum | ChEBI | | Docebenone | KEGG |
|
|---|
| Chemical Formula | C21H26O3 |
|---|
| Average Molecular Mass | 326.436 g/mol |
|---|
| Monoisotopic Mass | 326.188 g/mol |
|---|
| CAS Registry Number | 80809-81-0 |
|---|
| IUPAC Name | 2-(12-hydroxydodeca-5,10-diyn-1-yl)-3,5,6-trimethylcyclohexa-2,5-diene-1,4-dione |
|---|
| Traditional Name | docebenone |
|---|
| SMILES | CC1=C(C)C(=O)C(CCCCC#CCCCC#CCO)=C(C)C1=O |
|---|
| InChI Identifier | InChI=1S/C21H26O3/c1-16-17(2)21(24)19(18(3)20(16)23)14-12-10-8-6-4-5-7-9-11-13-15-22/h22H,5,7-10,12,14-15H2,1-3H3 |
|---|
| InChI Key | WDEABJKSGGRCQA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as long-chain fatty alcohols. These are fatty alcohols that have an aliphatic tail of 13 to 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohols |
|---|
| Direct Parent | Long-chain fatty alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Long chain fatty alcohol
- Quinone
- P-benzoquinone
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ox-2962000000-abd3ec831def4c16e180 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-1149000000-ee9cf43820a5790360b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aou-4291000000-b54f46e8a89615643eb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxs-9340000000-47c46820f60b02432052 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1039000000-28de35afe9200fac6a52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-2196000000-916c4c8cf284e02241ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-6191000000-c1d728bb77e7c43c52c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-066r-1294000000-4ae8b09de0e88fe84ae3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gbi-1950000000-76992e5deff2810694f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03du-3900000000-9b2e8f9c109f2b51a9c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-b47d84fc94860c2d0ace | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002b-2394000000-37690a164b5dafd39b87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0200-0920000000-6536a7083b0747b2b800 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0247732 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 1891 |
|---|
| ChEBI ID | 2340 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C01349 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|