| Synonyms | | Value | Source |
|---|
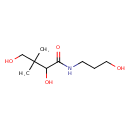
| DL-Panthenol | Kegg | | (+)-Panthenol | HMDB | | (+-)-Pantothenyl alcohol | HMDB | | (R)-2,4-Dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutanamide | HMDB | | 2,4-Dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutanamide | HMDB, MeSH | | Alcool DL-pantotenilico | HMDB | | Alcopan-250 | HMDB | | Bepanthen | HMDB, MeSH | | Bepanthene | HMDB | | Bepantol | HMDB | | Compnent OF ilopan-choline | HMDB | | D(+)-Panthenol | HMDB | | D(+)-Pantothenyl alcohol | HMDB | | D-(+)-2,4-Dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutyramide | HMDB | | D-(+)-Panthenol | HMDB | | D-(+)-Pantothenyl alcohol | HMDB | | D-P-a Injection | HMDB | | D-Panthenol | HMDB, MeSH | | D-Panthenol 50 | HMDB | | D-Pantothenol | HMDB | | D-Pantothenyl alcohol | HMDB | | Dexpantenol | HMDB | | Dexpanthenol | HMDB, MeSH | | Dexpanthenolum | HMDB | | dextro Pantothenyl alcohol | HMDB | | DL-Pantothenol | HMDB | | DL-Pantothenyl alcohol | HMDB | | Fancol DL | HMDB | | Ilopan | HMDB, MeSH | | Intrapan | HMDB | | Motilyn | HMDB | | N-Pantoyl-3-propanolamine | HMDB | | N-Pantoyl-propanolamine | HMDB | | Panadon | HMDB | | Pantenol | HMDB | | Pantenolo | HMDB | | Pantenyl | HMDB | | Panthenolum | HMDB | | Panthoderm | HMDB, MeSH | | Pantol | HMDB | | Pantothenyl alcohol | HMDB | | Pantothenylol | HMDB | | Penthenol | HMDB | | Provitamin b | HMDB | | Provitamin b5 | HMDB | | Synapan | HMDB | | Thenalton | HMDB | | Urupan | HMDB, MeSH | | Varitan | HMDB | | Zentinic | HMDB | | Jones brand OF dexpanthenol | MeSH, HMDB | | Panthenol jenapharm | MeSH, HMDB | | Repa-ophtal | MeSH, HMDB | | Roche consumer health brand OF dexpanthenol | MeSH, HMDB | | Ucee D | MeSH, HMDB | | Bioglan brand OF dexpanthenol | MeSH, HMDB | | Braun brand OF dexpanthenol | MeSH, HMDB | | Cassella-med brand OF dexpanthenol | MeSH, HMDB | | Febena brand OF dexpanthenol | MeSH, HMDB | | Jenapharm brand OF dexpanthenol | MeSH, HMDB | | Lichtenstein brand OF dexpanthenol | MeSH, HMDB | | Otriven dexpanthenol | MeSH, HMDB | | Pan rhinol | MeSH, HMDB | | Pan-ophtal | MeSH, HMDB | | Panthenol law | MeSH, HMDB | | Panthogenat | MeSH, HMDB | | Wund- und heilsalbe law | MeSH, HMDB | | Azupharma brand OF dexpanthenol | MeSH, HMDB | | Corneregel | MeSH, HMDB | | Dermapharm brand OF dexpanthenol | MeSH, HMDB | | Dexpanthenol heumann | MeSH, HMDB | | Heumann brand OF dexpanthenol | MeSH, HMDB | | Merck brand OF dexpanthenol | MeSH, HMDB | | Nasicur | MeSH, HMDB | | Panthenol lichtenstein | MeSH, HMDB | | Rhinoclir | MeSH, HMDB | | Roche nicholas brand OF dexpanthenol | MeSH, HMDB | | Roche brand OF dexpanthenol | MeSH, HMDB | | siozwo SANA | MeSH, HMDB | | Winzer brand OF dexpanthenol | MeSH, HMDB | | Artesan brand OF dexpanthenol | MeSH, HMDB | | LAW brand OF dexpanthenol | MeSH, HMDB | | Mann brand OF dexpanthenol | MeSH, HMDB | | Marolderm | MeSH, HMDB | | Merckle brand OF dexpanthenol | MeSH, HMDB | | NasenSpray ratiopharm panthenol | MeSH, HMDB | | Novartis brand OF dexpanthenol | MeSH, HMDB | | Panthenol braun | MeSH, HMDB | | Panthenol-ratiopharm | MeSH, HMDB | | Savage brand OF dexpanthenol | MeSH, HMDB | | CT-Arzneimittel brand OF dexpanthenol | MeSH, HMDB | | Panthenol von CT | MeSH, HMDB | | Ratiopharm brand OF dexpanthenol | MeSH, HMDB | | (+)-2,4-Dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutyramide | MeSH, HMDB | | Panthenol | MeSH, HMDB |
|
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0f8a-0911000000-fee05c47b9c7217624f0 | Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0uxr-0910000000-a8b75972916320313b23 | Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0f8a-0911000000-fee05c47b9c7217624f0 | Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0uxr-0910000000-a8b75972916320313b23 | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (4 TMS) | splash10-0f8a-1921000000-f1d6cd175435f0634e26 | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-0ar9-0940000000-60184a3322815ca33b8c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uk9-9800000000-8d98c51203986eae3367 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0kft-7972300000-9bb70f2e8bb63598a2d7 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0udi-0290000000-3a41e17463410b272ba9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0udi-0910000000-f4e5bccdc9f16d066c3c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0c09-0940000000-957d784682c5b786cb11 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05g0-9510000000-d2d9fa108eddb07460c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-9300000000-1f91c942e9bdd6b42509 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-c8a05e05963f03822948 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-2950000000-1783767ddbab04af52e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-5900000000-a70488ad2a04bfe4c1b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9200000000-09e799aa2d7432cd4670 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-3190000000-cf1eb0adc6af33ccd211 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-9100000000-ecfcc7613f38e3982719 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-9000000000-78f6619c4d8c33412f62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-3390000000-b4edf6f7542e2777ef5f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9200000000-7b2930b3e311cd0b08c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-c9bed78704f02ea6f7ea | Spectrum |
|
|---|