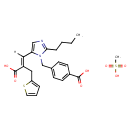
| (e)-2-Butyl-1-(p-carboxybenzyl)-alpha-2-thenylimidazole-5-acrylic acid, monomethanesulfonate | ChEBI |
| (e)-alpha-((2-Butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoic acid monomethanesulfonate | ChEBI |
| Eprosartan mesylate | ChEBI |
| Teveten | ChEBI |
| (e)-2-Butyl-1-(p-carboxybenzyl)-a-2-thenylimidazole-5-acrylate, monomethanesulfonate | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-a-2-thenylimidazole-5-acrylate, monomethanesulphonate | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-a-2-thenylimidazole-5-acrylic acid, monomethanesulfonic acid | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-a-2-thenylimidazole-5-acrylic acid, monomethanesulphonic acid | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-alpha-2-thenylimidazole-5-acrylate, monomethanesulfonate | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-alpha-2-thenylimidazole-5-acrylate, monomethanesulphonate | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-alpha-2-thenylimidazole-5-acrylic acid, monomethanesulfonic acid | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-alpha-2-thenylimidazole-5-acrylic acid, monomethanesulphonic acid | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-α-2-thenylimidazole-5-acrylate, monomethanesulfonate | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-α-2-thenylimidazole-5-acrylate, monomethanesulphonate | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-α-2-thenylimidazole-5-acrylic acid, monomethanesulfonic acid | Generator |
| (e)-2-Butyl-1-(p-carboxybenzyl)-α-2-thenylimidazole-5-acrylic acid, monomethanesulphonic acid | Generator |
| (e)-a-((2-Butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoate monomethanesulfonate | Generator |
| (e)-a-((2-Butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoate monomethanesulphonate | Generator |
| (e)-a-((2-Butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoic acid monomethanesulfonic acid | Generator |
| (e)-a-((2-Butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoic acid monomethanesulphonic acid | Generator |
| (e)-alpha-((2-Butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoate monomethanesulfonate | Generator |
| (e)-alpha-((2-Butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoate monomethanesulphonate | Generator |
| (e)-alpha-((2-Butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoic acid monomethanesulfonic acid | Generator |
| (e)-alpha-((2-Butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoic acid monomethanesulphonic acid | Generator |
| (e)-Α-((2-butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoate monomethanesulfonate | Generator |
| (e)-Α-((2-butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoate monomethanesulphonate | Generator |
| (e)-Α-((2-butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoic acid monomethanesulfonic acid | Generator |
| (e)-Α-((2-butyl-1-((4-carboxyphenyl)methyl)-1H-imidazol-5-yl)methylene)-2-thiophenepropanoic acid monomethanesulphonic acid | Generator |
| Eprosartan mesylic acid | Generator |
| Eprosartan methanesulfonic acid | Generator |
| Eprosartan methanesulphonate | Generator |
| Eprosartan methanesulphonic acid | Generator |
| SK And F 108566 | MeSH |
| Eprosartan | MeSH |