| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:42:29 UTC |
|---|
| Update Date | 2016-11-09 01:15:41 UTC |
|---|
| Accession Number | CHEM017871 |
|---|
| Identification |
|---|
| Common Name | Efonidipine |
|---|
| Class | Small Molecule |
|---|
| Description | Efonidipine is a calcium channel blocker of the _dihydropyridine class_, commercialized by Shionogi & Co. (Japan). Initially, it was marketed in 1995 under the trade name, _Landel_. The drug has been shown to block T-type in addition to L-type calcium channels [A7844, A32001]. It has also been studied in atherosclerosis and acute renal failure [A32001]. This drug is also known as NZ-105, and several studies have been done on its pharmacokinetics in animals [L1456]. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
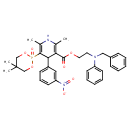
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Enfonidipine hydrochloride | MeSH | | 5-(5,5-Dimethyl-1,3,2-dioxaphosphorinan-2-yl)-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3-pyridinecarboxylic acid, 2-(phenyl(phenylmethyl)amino) ethyl ester, p-oxide, hydrochloride | MeSH | | Efonidipine HCL | ChEMBL | | 2-[Benzyl(phenyl)amino]ethyl 5-(5,5-dimethyl-2-oxo-1,3,2λ⁵-dioxaphosphinan-2-yl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid | Generator |
|
|---|
| Chemical Formula | C34H38N3O7P |
|---|
| Average Molecular Mass | 631.666 g/mol |
|---|
| Monoisotopic Mass | 631.245 g/mol |
|---|
| CAS Registry Number | 111011-63-3 |
|---|
| IUPAC Name | 2-[benzyl(phenyl)amino]ethyl 5-(5,5-dimethyl-2-oxo-1,3,2λ⁵-dioxaphosphinan-2-yl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylate |
|---|
| Traditional Name | efonidipine |
|---|
| SMILES | CC1=C(C(C2=CC=CC(=C2)[N+]([O-])=O)C(=C(C)N1)P1(=O)OCC(C)(C)CO1)C(=O)OCCN(CC1=CC=CC=C1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C34H38N3O7P/c1-24-30(33(38)42-19-18-36(28-15-9-6-10-16-28)21-26-12-7-5-8-13-26)31(27-14-11-17-29(20-27)37(39)40)32(25(2)35-24)45(41)43-22-34(3,4)23-44-45/h5-17,20,31,35H,18-19,21-23H2,1-4H3 |
|---|
| InChI Key | NSVFSAJIGAJDMR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylbenzamines. These are aromatic compounds consisting of a benzyl group that is N-linked to a benzamine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylmethylamines |
|---|
| Direct Parent | Phenylbenzamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylbenzamine
- Dihydropyridinecarboxylic acid derivative
- Nitrobenzene
- Benzylamine
- Nitroaromatic compound
- Tertiary aliphatic/aromatic amine
- Dialkylarylamine
- Aniline or substituted anilines
- Dihydropyridine
- Phosphonic acid diester
- Aralkylamine
- Hydropyridine
- Phosphonic acid ester
- Organophosphonic acid derivative
- Vinylogous amide
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- C-nitro compound
- Carboxylic acid ester
- Amino acid or derivatives
- Tertiary amine
- Organic nitro compound
- Allyl-type 1,3-dipolar organic compound
- Azacycle
- Secondary amine
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid derivative
- Organic 1,3-dipolar compound
- Secondary aliphatic amine
- Oxacycle
- Organic oxoazanium
- Enamine
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Organic zwitterion
- Amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Organophosphorus compound
- Carbonyl group
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-2000009000-bd26d50dddbe40bd1a8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-9000006000-adb4c04fcd90295c35cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-9011000000-7c9be6cd296b94d66f78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000x-1000009000-ac55941886d571a34ad6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0019-9300005000-1d798b40344ead2e9e3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9200000000-c414fd80a35c8cfc0cec | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB09235 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Efonidipine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 119171 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|