| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:42:06 UTC |
|---|
| Update Date | 2016-11-09 01:15:41 UTC |
|---|
| Accession Number | CHEM017862 |
|---|
| Identification |
|---|
| Common Name | Roxindole |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
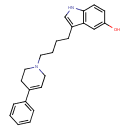
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-(4-(3,6-Dihydro-4-phenyl-1(2H)-pyridinyl)butyl)-1H-indol-5-ol | ChEBI | | 3-[4-(4-Phenyl-3,6-dihydro-2H-pyridin-1-yl)butyl]-1H-indol-5-ol | ChEBI | | Roxindol | ChEBI | | Roxindolum | ChEBI | | 5-Hydroxy-3-(4-(4-phenyl-1,2,3,6-tetrahydropyidyl)-1-butyl)-1-indole mesylate | MeSH | | Roxindole monohydrochloride | MeSH | | Roxindole mesylate | MeSH | | Roxindole hydrochloride | MeSH |
|
|---|
| Chemical Formula | C23H26N2O |
|---|
| Average Molecular Mass | 346.474 g/mol |
|---|
| Monoisotopic Mass | 346.205 g/mol |
|---|
| CAS Registry Number | 112192-04-8 |
|---|
| IUPAC Name | 3-[4-(4-phenyl-1,2,3,6-tetrahydropyridin-1-yl)butyl]-1H-indol-5-ol |
|---|
| Traditional Name | roxindole |
|---|
| SMILES | OC1=CC2=C(NC=C2CCCCN2CCC(=CC2)C2=CC=CC=C2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C23H26N2O/c26-21-9-10-23-22(16-21)20(17-24-23)8-4-5-13-25-14-11-19(12-15-25)18-6-2-1-3-7-18/h1-3,6-7,9-11,16-17,24,26H,4-5,8,12-15H2 |
|---|
| InChI Key | HGEYJZMMUGWEOT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxyindoles. These are organic compounds containing an indole moiety that carries a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Hydroxyindoles |
|---|
| Direct Parent | Hydroxyindoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyindole
- 3-alkylindole
- Indole
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Monocyclic benzene moiety
- Hydropyridine
- Benzenoid
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Amine
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00xr-0900000000-6ce3b89b8ab14fa08082 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0209000000-3226acdd91b5291a1408 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0946000000-38601482a48b861059ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052g-3901000000-0446d52c5777184dca58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-bd083b973e9aa7e410ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0109000000-023afbc3bfa7b48411ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pdi-1910000000-c4b76f34bab280fee083 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0257319 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Roxindole |
|---|
| Chemspider ID | 189880 |
|---|
| ChEBI ID | 48558 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|