| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:41:23 UTC |
|---|
| Update Date | 2016-11-09 01:15:41 UTC |
|---|
| Accession Number | CHEM017846 |
|---|
| Identification |
|---|
| Common Name | Temozolomide |
|---|
| Class | Small Molecule |
|---|
| Description | An imidazotetrazine that is 3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine which is substituted at positions 3, 4, and 8 by methyl, oxo, and carboxamide groups, respectively. A prodrug for MTIC (5-(3-methyltriaz-1-en-1-yl)-1H-imidazole-4-carboxamide, formed by spontaneous hydrolysis of temozolomide in the body), it is used as an oral alkylating agent for the treatment of newly diagnosed malignant glioblastoma multiforme (concomitantly with radiotherapy) and malignant melanoma. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
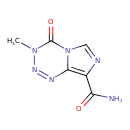
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,4-Dihydro-3-methyl-4-oxoimidazo(5,1-D)-1,2,3,5-tetrazine-8-carboxamide | ChEBI | | 3,4-Dihydro-3-methyl-4-oxoimidazo(5,1-D)-as-tetrazine-8-carboxamide | ChEBI | | 3-Methyl-4-oxo-3,4-dihydroimidazo(5,1-D)(1,2,3,5)tetrazine-8-carboxamide | ChEBI | | 8-Carbamoyl-3-methylimidazo(5,1-D)-1,2,3,5-tetrazin-4(3H)-one | ChEBI | | BRN 5547136 | ChEBI | | CCRG 81045 | ChEBI | | CCRG-81045 | ChEBI | | CCRIS 8996 | ChEBI | | m & b 39831 | ChEBI | | MB 39831 | ChEBI | | M&B 39831 | ChEBI | | Methazolastone | ChEBI | | NSC 362856 | ChEBI | | SCH 52365 | ChEBI | | Temodal | ChEBI | | Temodar | ChEBI | | Temozolomida | ChEBI | | Temozolomidum | ChEBI | | TMZ | ChEBI | | Temozolamide | HMDB | | m And b 39831 | HMDB | | m And b-39831 | HMDB | | TMZ-Bioshuttle | HMDB | | Temozolomide hexyl ester | HMDB | | TMZA-he | HMDB | | TMZ Bioshuttle | HMDB | | m And b39831 | HMDB |
|
|---|
| Chemical Formula | C6H6N6O2 |
|---|
| Average Molecular Mass | 194.151 g/mol |
|---|
| Monoisotopic Mass | 194.055 g/mol |
|---|
| CAS Registry Number | 85622-93-1 |
|---|
| IUPAC Name | 3-methyl-4-oxo-3H,4H-imidazo[4,3-d][1,2,3,5]tetrazine-8-carboxamide |
|---|
| Traditional Name | temozolomide |
|---|
| SMILES | CN1N=NC2=C(N=CN2C1=O)C(N)=O |
|---|
| InChI Identifier | InChI=1S/C6H6N6O2/c1-11-6(14)12-2-8-3(4(7)13)5(12)9-10-11/h2H,1H3,(H2,7,13) |
|---|
| InChI Key | BPEGJWRSRHCHSN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as imidazotetrazines. These are organic polycyclic compounds containing an imidazole ring fused to a tetrazine ring. Imidazole is 5-membered ring consisting of three carbon atoms, and two nitrogen centers at the 1- and 3-positions. Tetrazine is a six-membered aromatic heterocycle made up of four nitrogen atoms and a two carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazotetrazines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Imidazotetrazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Imidazotetrazine
- 2-heteroaryl carboxamide
- Imidazole-4-carbonyl group
- N-substituted imidazole
- Tetrazine
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Carboxamide group
- Primary carboxylic acid amide
- Carboxylic acid derivative
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-5900000000-c57261fbff38ae5dbab0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0a4r-9300000000-49f7261125953a02508c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-d9e1e206d7b06ccc479d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6t-0900000000-34157e6c0ae2f946b796 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-3900000000-39baca8459a2d7d69887 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-e01487cdb01c9cfbbca6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6x-2900000000-45ff34ca952c13e2e9e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9600000000-4719e8aa23ba0437bc9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-8368d930a3d0bfca1535 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ds-1900000000-01d724d7c70310752a11 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0929-3900000000-05ec3267ff2f648a63d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-eece1187427402b6806b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0900000000-a10f3dd11710d4812249 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-022c-3900000000-bb2a07e9cfc644fa8c80 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00853 |
|---|
| HMDB ID | HMDB0014991 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Temozolomide |
|---|
| Chemspider ID | 5201 |
|---|
| ChEBI ID | 72564 |
|---|
| PubChem Compound ID | 5394 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=22680781 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=22818211 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=23246370 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=23254891 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=23293540 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=23335050 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=23362460 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=23377829 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=23385995 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=23389760 | | 11. Yung WK: Temozolomide in malignant gliomas. Semin Oncol. 2000 Jun;27(3 Suppl 6):27-34. | | 12. Friedman HS, Kerby T, Calvert H: Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000 Jul;6(7):2585-97. | | 13. Mutter N, Stupp R: Temozolomide: a milestone in neuro-oncology and beyond? Expert Rev Anticancer Ther. 2006 Aug;6(8):1187-204. | | 14. Wick W, Platten M, Weller M: New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009 Feb;11(1):69-79. doi: 10.1215/15228517-2008-078. Epub 2008 Sep 4. | | 15. Trinh VA, Patel SP, Hwu WJ: The safety of temozolomide in the treatment of malignancies. Expert Opin Drug Saf. 2009 Jul;8(4):493-9. doi: 10.1517/14740330902918281 . | | 16. Villano JL, Seery TE, Bressler LR: Temozolomide in malignant gliomas: current use and future targets. Cancer Chemother Pharmacol. 2009 Sep;64(4):647-55. doi: 10.1007/s00280-009-1050-5. Epub 2009 Jun 19. | | 17. Meije Y, Lizasoain M, Garcia-Reyne A, Martinez P, Rodriguez V, Lopez-Medrano F, Juan RS, Lalueza A, Aguado JM: Emergence of cytomegalovirus disease in patients receiving temozolomide: report of two cases and literature review. Clin Infect Dis. 2010 Jun 15;50(12):e73-6. doi: 10.1086/653011. | | 18. Neyns B, Tosoni A, Hwu WJ, Reardon DA: Dose-dense temozolomide regimens: antitumor activity, toxicity, and immunomodulatory effects. Cancer. 2010 Jun 15;116(12):2868-77. doi: 10.1002/cncr.25035. |
|
|---|