| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:41:10 UTC |
|---|
| Update Date | 2016-11-09 01:15:40 UTC |
|---|
| Accession Number | CHEM017839 |
|---|
| Identification |
|---|
| Common Name | Tetrabenazine |
|---|
| Class | Small Molecule |
|---|
| Description | A drug formerly used as an antipsychotic but now used primarily in the symptomatic treatment of various hyperkinetic disorders. It is a monoamine depletor and used as symptomatic treatment of chorea associated with Huntington's disease. FDA approved on August 15, 2008. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
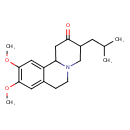
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2,4,6,7,11b-Hexahydro-3-isobutyl-9,10-dimethoxy-2H-benzo[a]quinolizin-2-one | ChEBI | | 2-oxo-3-Isobutyl-9,10-dimethoxy-1,2,3,4,6,7-hexahydro-11BH-benzo[a]quinolizine | ChEBI | | 2-oxo-3-Isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-benzoquinolizine | ChEBI | | Nitoman | Kegg | | Xenazine | Kegg | | Ro 1-9569 | HMDB | | TBZ | HMDB | | Tetra benazin | HMDB | | Tetrabenzaine | HMDB | | Tetrabenzine | HMDB | | Tetrabenazine orphan brand | HMDB | | Cambridge laboratories brand OF tetrabenazine | HMDB | | Orphan brand OF tetrabenazine | HMDB | | Roche brand OF tetrabenazine | HMDB | | Shire brand OF tetrabenazine | HMDB | | Tetrabenazine | MeSH |
|
|---|
| Chemical Formula | C19H27NO3 |
|---|
| Average Molecular Mass | 317.423 g/mol |
|---|
| Monoisotopic Mass | 317.199 g/mol |
|---|
| CAS Registry Number | 58-46-8 |
|---|
| IUPAC Name | 9,10-dimethoxy-3-(2-methylpropyl)-1H,2H,3H,4H,6H,7H,11bH-pyrido[2,1-a]isoquinolin-2-one |
|---|
| Traditional Name | tetrabenazine |
|---|
| SMILES | COC1=C(OC)C=C2C3CC(=O)C(CC(C)C)CN3CCC2=C1 |
|---|
| InChI Identifier | InChI=1S/C19H27NO3/c1-12(2)7-14-11-20-6-5-13-8-18(22-3)19(23-4)9-15(13)16(20)10-17(14)21/h8-9,12,14,16H,5-7,10-11H2,1-4H3 |
|---|
| InChI Key | MKJIEFSOBYUXJB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydroisoquinolines. These are tetrahydrogenated isoquinoline derivatives. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydroisoquinolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrahydroisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydroisoquinoline
- Anisole
- Alkyl aryl ether
- Piperidinone
- Aralkylamine
- Piperidine
- Benzenoid
- Ketone
- Tertiary amine
- Tertiary aliphatic amine
- Cyclic ketone
- Ether
- Azacycle
- Amine
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f6x-4292000000-399e16e38421335dc45d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0019000000-02d6ea72dcc42a44288e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-5269000000-f1240b2a09dcced1c899 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9430000000-cd11325cad96834bf22b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-484fd7bae49c0273c317 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-7059000000-cbb7a83dafd4d674e753 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uec-9270000000-5581f9758fcb31092e0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0009000000-0041714e9cb69e8982f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0039000000-7d6e398b5d4a2227026e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9281000000-0c9b09c6cde8e368ca06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-f61e4684d2f91b1d10d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0029000000-f7a121501bdf247273bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-1297000000-9bf4c9dd8adc56e35bd7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04844 |
|---|
| HMDB ID | HMDB0015592 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tetrabenazine |
|---|
| Chemspider ID | 5796 |
|---|
| ChEBI ID | 64028 |
|---|
| PubChem Compound ID | 6018 |
|---|
| Kegg Compound ID | C11168 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|