| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:41:05 UTC |
|---|
| Update Date | 2016-11-09 01:15:40 UTC |
|---|
| Accession Number | CHEM017836 |
|---|
| Identification |
|---|
| Common Name | Ritanserin |
|---|
| Class | Small Molecule |
|---|
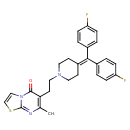
| Description | A thiazolopyrimidine that is 5H-[1,3]thiazolo[3,2-a]pyrimidin-5-one which is substituted at position 7 by a methyl group and at position 6 by a 2-{4-[bis(4-fluorophenyl)methylidene]piperidin-1-yl}ethyl group. A potent and long-acting seratonin (5-hydroxytryptamine, 5-HT) antagonist of the subtype 5-HT2 (Ki = 0.39 nM), it is used in the treatment of a variety of disorders including anxiety, depression and schizophrenia. It has little sedative action. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-(2-(4-(Bis(p-fluorophenyl)methylene)-piperidino)ethyl)-7-methyl-5H-thiazolo-(3,2-a)pyrimidin-5-one | ChEBI | | R 55,667 | ChEBI | | R-55667 | ChEBI | | Ritanserina | ChEBI | | Ritanserine | ChEBI | | Ritanserinum | ChEBI | | Tiserton | Kegg | | 6-(2-(4-(Bis(4-fluorophenyl)methylene)-1-piperidinyl)ethyl)-7-methyl-5H-thiazolo(3,2-a)pyrimidin-5-one | MeSH | | Ritanserin hydrochloride | MeSH | | Ritanserin tartrate | MeSH | | Ritanserin | MeSH |
|
|---|
| Chemical Formula | C27H25F2N3OS |
|---|
| Average Molecular Mass | 477.570 g/mol |
|---|
| Monoisotopic Mass | 477.169 g/mol |
|---|
| CAS Registry Number | 87051-43-2 |
|---|
| IUPAC Name | 6-(2-{4-[bis(4-fluorophenyl)methylidene]piperidin-1-yl}ethyl)-7-methyl-5H-[1,3]thiazolo[3,2-a]pyrimidin-5-one |
|---|
| Traditional Name | ritanserin |
|---|
| SMILES | CC1=C(CCN2CCC(CC2)=C(C2=CC=C(F)C=C2)C2=CC=C(F)C=C2)C(=O)N2C=CSC2=N1 |
|---|
| InChI Identifier | InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 |
|---|
| InChI Key | JUQLTPCYUFPYKE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Diphenylmethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylmethane
- Fluorobenzene
- Halobenzene
- Pyrimidone
- Aralkylamine
- Aryl fluoride
- Aryl halide
- Piperidine
- Pyrimidine
- Thiazole
- Heteroaromatic compound
- Azole
- Tertiary aliphatic amine
- Tertiary amine
- Lactam
- Azacycle
- Organoheterocyclic compound
- Organic oxide
- Organofluoride
- Organohalogen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Amine
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f6t-3290000000-bc5d102863d190032aed | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0fc3-2900000000-aeed8cd6efbb3b93af8e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-2900000000-e63814b1ab90bb7dbff0 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0fc3-2900000000-aeed8cd6efbb3b93af8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0120900000-2b497f82b7be60923129 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002g-0691400000-0c8177d3e5ab3969c8e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-054o-4964000000-91e06533d512fbdbbae9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000900000-8551e56e48dfdf64e171 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00o0-0120900000-36880e5ac2306f556ef6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-8390300000-ac0d1ec8fba9b1d3ebac | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB12693 |
|---|
| HMDB ID | HMDB0257243 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ritanserin |
|---|
| Chemspider ID | 4896 |
|---|
| ChEBI ID | 64195 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|