| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:41:00 UTC |
|---|
| Update Date | 2016-11-09 01:15:40 UTC |
|---|
| Accession Number | CHEM017834 |
|---|
| Identification |
|---|
| Common Name | Metergoline |
|---|
| Class | Small Molecule |
|---|
| Description | An ergoline alkaloid that is the N-benzyloxycarbonyl derivative of lysergamine. A 5-HT2 antagonist. Also 5-HT1 antagonist and 5-HT1D ligand. Has moderate affinity for 5-HT6 and high affinity for 5-HT7. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
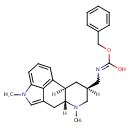
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (((8-beta)-1,6-Dimethylergolin-8-yl)methyl)carbamic acid benzyl ester | ChEBI | | (((8-beta)-1,6-Dimethylergolin-8-yl)methyl)carbamic acid phenylmethyl ester | ChEBI | | (+)-N-(Carboxy)-1-methyl-9,10-dihydrolysergamine benzyl ester | ChEBI | | 1,6-Dimethyl-8-beta-carbobenzyloxaminomethyl-10-alpha-ergoline | ChEBI | | 1-Methyl-8-beta-carbobenzyloxyaminomethyl-10-alpha-ergoline | ChEBI | | 1-Methyl-N-carbobenzyloxydihydro-D-lysergamin | ChEBI | | D-8-beta-((Carbobenzoxyamino)methyl)-1,6-dimethyl-10-alpha-ergoline | ChEBI | | D-8-beta-((Carboxyamino)methyl)-1,6-dimethylergoline benzyl ester | ChEBI | | Metergolin | ChEBI | | Metergolina | ChEBI | | Metergolinum | ChEBI | | (((8-b)-1,6-Dimethylergolin-8-yl)methyl)carbamate benzyl ester | Generator | | (((8-b)-1,6-Dimethylergolin-8-yl)methyl)carbamic acid benzyl ester | Generator | | (((8-beta)-1,6-Dimethylergolin-8-yl)methyl)carbamate benzyl ester | Generator | | (((8-Β)-1,6-dimethylergolin-8-yl)methyl)carbamate benzyl ester | Generator | | (((8-Β)-1,6-dimethylergolin-8-yl)methyl)carbamic acid benzyl ester | Generator | | (((8-b)-1,6-Dimethylergolin-8-yl)methyl)carbamate phenylmethyl ester | Generator | | (((8-b)-1,6-Dimethylergolin-8-yl)methyl)carbamic acid phenylmethyl ester | Generator | | (((8-beta)-1,6-Dimethylergolin-8-yl)methyl)carbamate phenylmethyl ester | Generator | | (((8-Β)-1,6-dimethylergolin-8-yl)methyl)carbamate phenylmethyl ester | Generator | | (((8-Β)-1,6-dimethylergolin-8-yl)methyl)carbamic acid phenylmethyl ester | Generator | | 1,6-Dimethyl-8-b-carbobenzyloxaminomethyl-10-a-ergoline | Generator | | 1,6-Dimethyl-8-β-carbobenzyloxaminomethyl-10-α-ergoline | Generator | | 1-Methyl-8-b-carbobenzyloxyaminomethyl-10-a-ergoline | Generator | | 1-Methyl-8-β-carbobenzyloxyaminomethyl-10-α-ergoline | Generator | | D-8-b-((Carbobenzoxyamino)methyl)-1,6-dimethyl-10-a-ergoline | Generator | | D-8-Β-((carbobenzoxyamino)methyl)-1,6-dimethyl-10-α-ergoline | Generator | | D-8-b-((Carboxyamino)methyl)-1,6-dimethylergoline benzyl ester | Generator | | D-8-Β-((carboxyamino)methyl)-1,6-dimethylergoline benzyl ester | Generator | | Metergoline teofarma brand | MeSH | | Liserdol | MeSH | | Methergoline | MeSH | | Teofarma brand OF metergoline | MeSH |
|
|---|
| Chemical Formula | C25H29N3O2 |
|---|
| Average Molecular Mass | 403.526 g/mol |
|---|
| Monoisotopic Mass | 403.226 g/mol |
|---|
| CAS Registry Number | 17692-51-2 |
|---|
| IUPAC Name | N-{[(2R,4R,7R)-6,11-dimethyl-6,11-diazatetracyclo[7.6.1.0²,⁷.0¹²,¹⁶]hexadeca-1(16),9,12,14-tetraen-4-yl]methyl}(benzyloxy)carboximidic acid |
|---|
| Traditional Name | methergoline |
|---|
| SMILES | [H][C@]1(CN=C(O)OCC2=CC=CC=C2)CN(C)[C@]2([H])CC3=CN(C)C4=CC=CC(=C34)[C@@]2([H])C1 |
|---|
| InChI Identifier | InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 |
|---|
| InChI Key | WZHJKEUHNJHDLS-QTGUNEKASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indoloquinolines. These are polycyclic aromatic compounds containing an indole fused to a quinoline. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Indoloquinolines |
|---|
| Direct Parent | Indoloquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ergoline skeleton
- Indoloquinoline
- Benzoquinoline
- Pyrroloquinoline
- Benzyloxycarbonyl
- N-alkylindole
- 3-alkylindole
- Indole
- Indole or derivatives
- Isoindole or derivatives
- Alkaloid or derivatives
- Aralkylamine
- Benzenoid
- Monocyclic benzene moiety
- N-methylpyrrole
- Substituted pyrrole
- Piperidine
- Heteroaromatic compound
- Carbamic acid ester
- Pyrrole
- Carbonic acid derivative
- Tertiary amine
- Tertiary aliphatic amine
- Azacycle
- Organonitrogen compound
- Organic oxide
- Amine
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0ir0-4794200000-d04091d908ab86f9807e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0ir0-4794200000-d04091d908ab86f9807e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0191300000-4835b4de6449fef92fac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fr6-3190000000-0d779d001d5c1019b1e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01bi-1490000000-2c9b03a38c2e1316c36b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1290200000-b57626c1f72345f0dc0f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9370000000-56877b695eaa70a67a42 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9470000000-a8cb594fb31952430f98 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13520 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Metergoline |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 64216 |
|---|
| PubChem Compound ID | 28693 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|