| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:40:58 UTC |
|---|
| Update Date | 2016-11-09 01:15:40 UTC |
|---|
| Accession Number | CHEM017833 |
|---|
| Identification |
|---|
| Common Name | Levosulpiride |
|---|
| Class | Small Molecule |
|---|
| Description | An optically active form of sulpiride having (S)-configuration. The active enantiomer of the racemic drug sulpiride. Selective D2-like dopamine antagonist (Ki values are ~ 0.015. ~ 0.013, 1, ~ 45 and ~ 77 muM at D2, D3, D4, D1 and D5 receptors respectively). |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
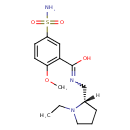
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Sulpiride | ChEBI | | (-)-N-(((S)-1-Ethyl-2-pyrrolidinyl)methyl)-5-sulfamoyl-O-anisamide | ChEBI | | (S)-(-)-5-Aminosulfonyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxybenzamide | ChEBI | | (S)-(-)-N-((1-Ethyl-2-pyrrolidinyl)methyl)-5-sulfamoyl-O-anisamide | ChEBI | | (S)-Sulpiride | ChEBI | | Levosulpirida | ChEBI | | Levosulpiride | ChEBI | | Levosulpiridum | ChEBI | | S-(-)-N-(1-Ethyl-2-pyrrolidinomethyl)-2-methoxy-5-sulfamoylebenzamide | ChEBI | | Levopraid | Kegg | | (-)-N-(((S)-1-Ethyl-2-pyrrolidinyl)methyl)-5-sulphamoyl-O-anisamide | Generator | | (S)-(-)-5-Aminosulphonyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxybenzamide | Generator | | (S)-(-)-N-((1-Ethyl-2-pyrrolidinyl)methyl)-5-sulphamoyl-O-anisamide | Generator | | S-(-)-N-(1-Ethyl-2-pyrrolidinomethyl)-2-methoxy-5-sulphamoylebenzamide | Generator | | N-(((S)-1-Ethyl-2-pyrrolidinyl)methyl)-5-sulfamoyl-O-anisamide | MeSH | | N-[[(2S)-1-Ethylpyrrolidin-2-yl]methyl]-2-methoxy-5-sulphamoylbenzamide | Generator |
|
|---|
| Chemical Formula | C15H23N3O4S |
|---|
| Average Molecular Mass | 341.430 g/mol |
|---|
| Monoisotopic Mass | 341.141 g/mol |
|---|
| CAS Registry Number | 23672-07-3 |
|---|
| IUPAC Name | N-{[(2S)-1-ethylpyrrolidin-2-yl]methyl}-2-methoxy-5-sulfamoylbenzene-1-carboximidic acid |
|---|
| Traditional Name | S-(-)-sulpiride |
|---|
| SMILES | [H][C@@]1(CN=C(O)C2=C(OC)C=CC(=C2)S(N)(=O)=O)CCCN1CC |
|---|
| InChI Identifier | InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21)/t11-/m0/s1 |
|---|
| InChI Key | BGRJTUBHPOOWDU-NSHDSACASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzenesulfonamides. These are organic compounds containing a sulfonamide group that is S-linked to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonamides |
|---|
| Direct Parent | Benzenesulfonamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzenesulfonamide
- Benzamide
- Benzenesulfonyl group
- Benzoic acid or derivatives
- Phenoxy compound
- Anisole
- Benzoyl
- Methoxybenzene
- Phenol ether
- Alkyl aryl ether
- N-alkylpyrrolidine
- Organosulfonic acid amide
- Pyrrolidine
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Aminosulfonyl compound
- Sulfonyl
- Tertiary aliphatic amine
- Tertiary amine
- Amino acid or derivatives
- Carboxamide group
- Secondary carboxylic acid amide
- Ether
- Carboxylic acid derivative
- Azacycle
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organosulfur compound
- Organic nitrogen compound
- Amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ox-0519000000-709a0cc07372655e216e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-2931000000-a4feed04814e6a775edb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01qm-9300000000-41411c150a46fffcffd5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0019000000-908ca17fb0c5d8ea4da3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-6936000000-913d80650e823e5a057c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9210000000-e9d0ec345582ac120e35 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Levosulpiride |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 64119 |
|---|
| PubChem Compound ID | 688272 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|