| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:40:57 UTC |
|---|
| Update Date | 2016-11-09 01:15:40 UTC |
|---|
| Accession Number | CHEM017832 |
|---|
| Identification |
|---|
| Common Name | AMI-193 |
|---|
| Class | Small Molecule |
|---|
| Description | An azaspiro compound that consists of 1,3,8-triazaspirodecan-4-one having a phenyl group attached to N-1 and a 3-(4-fluorophenoxy)propyl attached to N-8. Selective 5-HT antagonist, which binds to 5-HT2 sites as potently as spiperone but has lower affinity for 5-HT2C receptors. Also a high affinity D2 receptor antagonist (Ki = 3 nM). Lacks the disruptive effect of spiperone on animal behaviour. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| AMI-193 | ChEBI | | Espiramida | ChEBI | | Spiramidum | ChEBI | | Spiramide hydrochloride | MeSH | | Spiramide monohydrochloride | MeSH | | 8-(3-(4-Fluorophenoxy) propyl)-1-phenyl-1,3,8-triazaspiro(4, 5)decan-4-one | MeSH |
|
|---|
| Chemical Formula | C22H26FN3O2 |
|---|
| Average Molecular Mass | 383.467 g/mol |
|---|
| Monoisotopic Mass | 383.201 g/mol |
|---|
| CAS Registry Number | 510-74-7 |
|---|
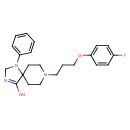
| IUPAC Name | 8-[3-(4-fluorophenoxy)propyl]-1-phenyl-1,3,8-triazaspiro[4.5]dec-3-en-4-ol |
|---|
| Traditional Name | 8-[3-(4-fluorophenoxy)propyl]-1-phenyl-1,3,8-triazaspiro[4.5]dec-3-en-4-ol |
|---|
| SMILES | OC1=NCN(C2=CC=CC=C2)C11CCN(CCCOC2=CC=C(F)C=C2)CC1 |
|---|
| InChI Identifier | InChI=1S/C22H26FN3O2/c23-18-7-9-20(10-8-18)28-16-4-13-25-14-11-22(12-15-25)21(27)24-17-26(22)19-5-2-1-3-6-19/h1-3,5-10H,4,11-17H2,(H,24,27) |
|---|
| InChI Key | FJUKDAZEABGEIH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as azaspirodecane derivatives. These are organic compounds containing a spirodecane moiety with at least one nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azaspirodecane derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Azaspirodecane derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Azaspirodecane
- Phenoxy compound
- Aniline or substituted anilines
- Dialkylarylamine
- Phenol ether
- Alkyl aryl ether
- Fluorobenzene
- Halobenzene
- Aryl fluoride
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Piperidine
- Cyclic carboximidic acid
- 3-imidazoline
- Tertiary aliphatic amine
- Tertiary amine
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Ether
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0129000000-7785d7f6c7c715fcf4e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6x-1594000000-fa8dd9040f7d435a8ec7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0j4l-6940000000-1bb73a10a7e899831207 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0309000000-e6428fe7a5066906e8f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03e9-0924000000-c30dbbe028528e3c34e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-2900000000-e639f90b1bcd28122952 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Spiramide |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 64207 |
|---|
| PubChem Compound ID | 68186 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|