| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:36:16 UTC |
|---|
| Update Date | 2016-11-09 01:15:39 UTC |
|---|
| Accession Number | CHEM017752 |
|---|
| Identification |
|---|
| Common Name | Dihydro-alpha-terpineol |
|---|
| Class | Small Molecule |
|---|
| Description | Menthanol is found in citrus. Menthanol is present in lemon and spearmint oil |
|---|
| Contaminant Sources | - FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
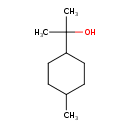
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(1-Hydroxy-1-methylethyl)-4-methylcyclohexane | HMDB | | 1-Methyl-4-isopropylcyclohexane-8-ol | HMDB | | 2-(4-Methylcyclohexyl)-2-propanol | HMDB | | a,a,4-Trimethylcyclohexanemethanol, 9ci | HMDB | | alpha -Dihydroterpineol | HMDB | | alpha,alpha,4-Trimethyl-cis-cyclohexanemethanol | HMDB | | alpha,alpha,4-Trimethyl-cyclohexanemethanol | HMDB | | alpha,alpha,4-Trimethyl-trans-cyclohexanemethanol | HMDB | | alpha,alpha,4-Trimethylcyclohexanemethanol | HMDB | | cis-alpha,alpha,4-Trimethylcyclohexanemethanol | HMDB | | dihydro-a-Terpineol | HMDB | | dihydro-alpha -Terpineol | HMDB | | dihydro-alpha-Terpineol | HMDB | | dihydro-Terpineol | HMDB | | trans-(1)-alpha,alpha,4-Trimethylcyclohexanemethanol | HMDB | | trans-2-(4-Methylcyclohexyl)isopropanol | HMDB | | trans-alpha,alpha,4-Trimethylcyclohexanemethanol | HMDB | | trans-P-Menthan-8-ol | HMDB | | 1-Methyl-4-isopropylcyclohexan-8-ol | MeSH, HMDB |
|
|---|
| Chemical Formula | C10H20O |
|---|
| Average Molecular Mass | 156.265 g/mol |
|---|
| Monoisotopic Mass | 156.151 g/mol |
|---|
| CAS Registry Number | 498-81-7 |
|---|
| IUPAC Name | 2-(4-methylcyclohexyl)propan-2-ol |
|---|
| Traditional Name | 2-(4-methylcyclohexyl)propan-2-ol |
|---|
| SMILES | CC1CCC(CC1)C(C)(C)O |
|---|
| InChI Identifier | InChI=1S/C10H20O/c1-8-4-6-9(7-5-8)10(2,3)11/h8-9,11H,4-7H2,1-3H3 |
|---|
| InChI Key | UODXCYZDMHPIJE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Menthane monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-menthane monoterpenoid
- Monocyclic monoterpenoid
- Tertiary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9200000000-0328cf5b09222f710cb3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01qi-9620000000-55b1d5a76f67f5051e49 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-1900000000-1280c251635b19021ed3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052k-9600000000-77cee19a7027720fcea3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-9200000000-d9945a1290f2ac0d3129 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-f6069eab4add4d4fdeb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4j-4900000000-7f7afd1f9b3a65c01617 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9400000000-01d7abacc51972b908d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052b-9400000000-8ca5e8adfdf8a9afa671 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-007w-9200000000-0b60d461d0a674c803b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9000000000-66eb53d5f1976c251c95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-f6034c6a8245c68a37ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-e2ccc681fac58db6d602 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-4900000000-21c08d7b84fbe1086325 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0034717 |
|---|
| FooDB ID | FDB013253 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00010899 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9926 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10353 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|