| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:30:09 UTC |
|---|
| Update Date | 2016-11-09 01:15:38 UTC |
|---|
| Accession Number | CHEM017626 |
|---|
| Identification |
|---|
| Common Name | Dasatinib |
|---|
| Class | Small Molecule |
|---|
| Description | Dasatinib is an oral dual BCR/ABL and Src family tyrosine kinase inhibitor approved for use in patients with chronic myelogenous leukemia (CML). The main targets of Dasatinib, are BCRABL, SRC, Ephrins and GFR. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
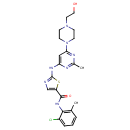
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Anh. dasatinib | ChEBI | | Anhydrous dasatinib | ChEBI | | BMS Dasatinib | ChEBI | | BMS-354825 | ChEBI | | Dasatinib (anh.) | ChEBI | | Dasatinibum | ChEBI | | N-(2-CHLORO-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide | ChEBI | | (18F)-N-(2-Chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide | HMDB | | N-(2-Chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide | HMDB | | Sprycel | HMDB |
|
|---|
| Chemical Formula | C22H26ClN7O2S |
|---|
| Average Molecular Mass | 488.006 g/mol |
|---|
| Monoisotopic Mass | 487.156 g/mol |
|---|
| CAS Registry Number | 302962-49-8 |
|---|
| IUPAC Name | N-(2-chloro-6-methylphenyl)-2-({6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)-1,3-thiazole-5-carboxamide |
|---|
| Traditional Name | dasatinib |
|---|
| SMILES | CC1=NC(NC2=NC=C(S2)C(=O)NC2=C(C)C=CC=C2Cl)=CC(=N1)N1CCN(CCO)CC1 |
|---|
| InChI Identifier | InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) |
|---|
| InChI Key | ZBNZXTGUTAYRHI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acid amides. These are amide derivatives of alpha amino acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acid amides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid amide
- Amphetamine or derivatives
- Phenylpropane
- Monocyclic benzene moiety
- Fatty amide
- Fatty acyl
- Benzenoid
- N-acyl-amine
- Carboxamide group
- Secondary carboxylic acid amide
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Primary amine
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4j-3104900000-19c063b36c545a5eb85a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00r7-3101490000-d274cb5f2cc0589526f6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-000m-0390000000-8a413cb9e53d14cbe10a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-000i-0011900000-4dac51b915117532ebe0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000l-0521900000-f036db5f090dc721c061 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0945000000-1344a7f6fa72fda9679c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-1941000000-7dd50ec0e4437edbdddb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-06ri-0293600000-003749b25fb59315664f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066v-1292100000-f90b69c609d80d3f92a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-3390000000-0e74e360d8dc98f4adc8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000900000-e70e9316e0f943fd209d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0001900000-009c64fdb2ae58cfd0fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f72-7395800000-2de6701f9f023ff4fc04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-25ba0df4df3ee5cf8e03 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fs9-4112900000-d8d24063d0379ec5b337 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-4192000000-f29c2b38b827f08243f6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01254 |
|---|
| HMDB ID | HMDB0015384 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Dasatinib |
|---|
| Chemspider ID | 2323020 |
|---|
| ChEBI ID | 49375 |
|---|
| PubChem Compound ID | 3062316 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O'Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir MA, Iyer V, Chen TT, Huang F, Decillis AP, Sawyers CL: Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006 Jun 15;354(24):2531-41. | | 2. Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, Pang S, Shen DR, Fang Q, de Fex HF, McIntyre KW, Shuster DJ, Gillooly KM, Behnia K, Schieven GL, Wityak J, Barrish JC: 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1- piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med Chem. 2006 Nov 16;49(23):6819-32. | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=16775234 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=17154512 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=18020922 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=18784745 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=18797457 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=18823558 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=19494352 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=19502192 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=19640584 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=21226671 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=22411867 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=22740998 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=22992064 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=23065516 |
|
|---|