| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:28:32 UTC |
|---|
| Update Date | 2016-11-09 01:15:37 UTC |
|---|
| Accession Number | CHEM017582 |
|---|
| Identification |
|---|
| Common Name | Oxymetazoline |
|---|
| Class | Small Molecule |
|---|
| Description | A direct acting sympathomimetic used as a vasoconstrictor to relieve nasal congestion. (From Martindale, The Extra Pharmacopoeia, 30th ed, p1251) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(4-Tert-butyl-2,6-dimethyl-3-hydroxybenzyl)-2-imidazoline | ChEBI | | 3-[(4,5-Dihydro-1H-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethylphenol | ChEBI | | 6-t-Butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol | ChEBI | | 6-Tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol | ChEBI | | Oxymetazolina | ChEBI | | Oxymetazolinum | ChEBI | | Afrin | Kegg | | Operil | Kegg | | Oximetazolinum | HMDB | | Oxymetazoline hydrochloride crystalline | HMDB | | Oxymethazoline | HMDB | | Oxymetozoline | HMDB | | Hydrochloride, oxymetazoline | HMDB | | Oxymetazoline hydrochloride | HMDB |
|
|---|
| Chemical Formula | C16H24N2O |
|---|
| Average Molecular Mass | 260.375 g/mol |
|---|
| Monoisotopic Mass | 260.189 g/mol |
|---|
| CAS Registry Number | 1491-59-4 |
|---|
| IUPAC Name | 6-tert-butyl-3-(4,5-dihydro-1H-imidazol-2-ylmethyl)-2,4-dimethylphenol |
|---|
| Traditional Name | oxymetazoline |
|---|
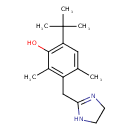
| SMILES | CC1=CC(=C(O)C(C)=C1CC1=NCCN1)C(C)(C)C |
|---|
| InChI Identifier | InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) |
|---|
| InChI Key | WYWIFABBXFUGLM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xylenols. These are aromatic compounds that contain a xylene moiety, which is a monocyclic benzene carrying exactly two methyl groups, and at least one hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Xylenes |
|---|
| Direct Parent | Xylenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xylenol
- Phenylpropane
- M-xylene
- O-cresol
- P-cresol
- Phenol
- Imidolactam
- 2-imidazoline
- Amidine
- Carboxylic acid amidine
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Organic nitrogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004m-3970000000-ee2ec321a55bc78173e1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01di-9847000000-0444f6bbf25ec5a41405 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0090000000-c488f3b0f9552a4b7dfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1290000000-a18cdab404c55b4e4497 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-7960000000-1c8f2a11df7638b325df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-fd2fa239fe46b8ca41c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1090000000-0e91b1578606ee50752f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01r6-4890000000-3e6c50f85057e4ad43d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0190000000-d99fec2889c2b1f7a321 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dr-1970000000-c0fcd30c275a95baee23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0596-6910000000-b96b7ef48bcbbf6173b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-7c4aa654b272455d2904 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-0970000000-1e55ae3560aa333e78fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ac-0930000000-4066d357ff89a3ee1206 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00935 |
|---|
| HMDB ID | HMDB0015070 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Oxymetazoline |
|---|
| Chemspider ID | 4475 |
|---|
| ChEBI ID | 7862 |
|---|
| PubChem Compound ID | 4636 |
|---|
| Kegg Compound ID | C07363 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|