| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:28:23 UTC |
|---|
| Update Date | 2016-11-09 01:15:37 UTC |
|---|
| Accession Number | CHEM017577 |
|---|
| Identification |
|---|
| Common Name | Megestrol acetate |
|---|
| Class | Small Molecule |
|---|
| Description | 17-Hydroxy-6-methylpregna-3,6-diene-3,20-dione. A progestational hormone used most commonly as the acetate ester. As the acetate, it is more potent than progesterone both as a progestagen and as an ovulation inhibitor. It has also been used in the palliative treatment of breast cancer. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
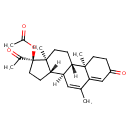
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Megace | Kegg | | Megestrol acetic acid | Generator | | Mestrel | MeSH | | Nu megestrol | MeSH | | NuMegestrol | MeSH | | apo Megestrol | MeSH | | Lin megestrol | MeSH | | Nu-megestrol | MeSH | | Nu-pharm brand OF megestrol acetate | MeSH | | LinMegestrol | MeSH | | Prasfarma brand OF megestrol acetate | MeSH | | apo-Megestrol | MeSH | | ApoMegestrol | MeSH | | Bristol-myers squibb brand OF megestrol acetate | MeSH | | Maygace | MeSH | | Borea | MeSH | | Linson pharma brand OF megestrol acetate | MeSH | | Megestat | MeSH | | Megostat | MeSH | | Apotex brand OF megestrol acetate | MeSH | | Bristol-myers brand OF megestrol acetate | MeSH | | Lin-megestrol | MeSH | | Acetate, megestrol | MeSH | | Bristol myers brand OF megestrol acetate | MeSH | | Lemery brand OF megestrol acetate | MeSH | | Nu pharm brand OF megestrol acetate | MeSH | | Bristol myers squibb brand OF megestrol acetate | MeSH | | Madaus brand OF megestrol acetate | MeSH | | Megefren | MeSH | | Squibb brand OF megestrol acetate | MeSH |
|
|---|
| Chemical Formula | C24H32O4 |
|---|
| Average Molecular Mass | 384.516 g/mol |
|---|
| Monoisotopic Mass | 384.230 g/mol |
|---|
| CAS Registry Number | 595-33-5 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14R,15S)-14-acetyl-2,8,15-trimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-6,8-dien-14-yl acetate |
|---|
| Traditional Name | DMAP |
|---|
| SMILES | [H][C@@]12CC[C@](OC(C)=O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=C(C)C2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C24H32O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h12-13,18-20H,6-11H2,1-5H3/t18-,19+,20+,22-,23+,24+/m1/s1 |
|---|
| InChI Key | RQZAXGRLVPAYTJ-GQFGMJRRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- Steroid ester
- 3-oxosteroid
- Oxosteroid
- Cyclohexenone
- Alpha-acyloxy ketone
- Ketone
- Carboxylic acid ester
- Cyclic ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organic oxide
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - C21 steroids (gluco/mineralocorticoids, progestogens) and derivatives (C08151 )
- C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030118 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-002r-0379000000-6d73cd082b939b89d5ce | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-060r-2890000000-fa2ec02a8c51a731cc7b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-00n0-0497000000-046272fbcad7d1d41298 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000l-0009000000-59a0980387ea4612f8f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00mo-0059000000-5a746fc4d3061ff006bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v4i-1981000000-0886e38a4167a0cb7c1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001l-1009000000-3ddafe06b118017c8af5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000x-2029000000-ae6024f2a71c21be2d49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6v-9067000000-723980bd7c6c229868b9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00351 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Megestrol acetate |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11683 |
|---|
| Kegg Compound ID | C08151 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|