| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:28:15 UTC |
|---|
| Update Date | 2016-11-09 01:15:37 UTC |
|---|
| Accession Number | CHEM017573 |
|---|
| Identification |
|---|
| Common Name | Glimepiride |
|---|
| Class | Small Molecule |
|---|
| Description | First introduced in 1995, glimepiride is a member of the second-generation sulfonylurea (SU) drug class used for the management of type 2 diabetes mellitus (T2DM) to improve glycemic control. Type 2 diabetes is a metabolic disorder with increasing prevalences worldwide; it is characterized by insulin resistance in accordance with progressive β cell failure and long-term microvascular and macrovascular complications that lead to co-morbidities and mortalities. Sulfonylureas are one of the insulin secretagogues widely used for the management of type 2 diabetes to lower blood glucose levels. The main effect of SUs is thought to be effective when residual pancreatic β-cells are present, as they work by stimulating the release of insulin from the pancreatic beta cells and they are also thought to exert extra-pancreatic effects, such as increasing the insulin-mediated peripheral glucose uptake.
Glimepiride works by stimulating the secretion of insulin granules from pancreatic islet beta cells by blocking ATP-sensitive potassium channels (KATP channels) and causing depolarization of the beta cells. Compared to , another second SU drug, glimepiride has a longer duration of action. It is sometimes classified as a third-generation SU because it has larger substitutions than other second-generation SUs. Compared to other SUs, glimepiride was associated with a lower risk of developing hypoglycemia and weight gain in clinical trials as well as fewer cardiovascular effects than other SUs due to minimal effects on ischemic preconditioning of cardiac myocytes. It is effective in reducing fasting plasma glucose, postprandial glucose, and glycosylated hemoglobin levels and is considered to be a useful, cost-effective treatment option for managing type 2 diabetes mellitus. Glimepiride was approved by the Food and Drug Administration (FDA) in the United States in 1995 for the treatment of T2DM. It is commonly marketed under the brand name Glimepiride as oral tablets and is typically administered once daily. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-((p-(2-(3-Ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl)phenyl)sulfonyl)-3-(trans-4-methylcyclohexyl)urea | ChEBI | | 1-{[4-(2-{[(3-ethyl-4-methyl-2-oxo-2,5-dihydro-1H-pyrrol-1-yl)carbonyl]amino}ethyl)phenyl]sulfonyl}-3-(trans-4-methylcyclohexyl)urea | ChEBI | | Amaryl | ChEBI | | Gimepiride | ChEBI | | Glimepirida | ChEBI | | Glimepiridum | ChEBI | | 1-((p-(2-(3-Ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl)phenyl)sulphonyl)-3-(trans-4-methylcyclohexyl)urea | Generator | | 1-{[4-(2-{[(3-ethyl-4-methyl-2-oxo-2,5-dihydro-1H-pyrrol-1-yl)carbonyl]amino}ethyl)phenyl]sulphonyl}-3-(trans-4-methylcyclohexyl)urea | Generator |
|
|---|
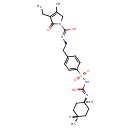
| Chemical Formula | C24H34N4O5S |
|---|
| Average Molecular Mass | 490.620 g/mol |
|---|
| Monoisotopic Mass | 490.225 g/mol |
|---|
| CAS Registry Number | 93479-97-1 |
|---|
| IUPAC Name | 3-ethyl-4-methyl-2-oxo-N-(2-{4-[({[(1r,4r)-4-methylcyclohexyl]-C-hydroxycarbonimidoyl}amino)sulfonyl]phenyl}ethyl)-2,5-dihydro-1H-pyrrole-1-carboximidic acid |
|---|
| Traditional Name | glimepiride |
|---|
| SMILES | [H][C@]1(C)CC[C@@]([H])(CC1)N=C(O)NS(=O)(=O)C1=CC=C(CCN=C(O)N2CC(C)=C(CC)C2=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C24H34N4O5S/c1-4-21-17(3)15-28(22(21)29)24(31)25-14-13-18-7-11-20(12-8-18)34(32,33)27-23(30)26-19-9-5-16(2)6-10-19/h7-8,11-12,16,19H,4-6,9-10,13-15H2,1-3H3,(H,25,31)(H2,26,27,30)/t16-,19- |
|---|
| InChI Key | WIGIZIANZCJQQY-RUCARUNLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzenesulfonamides. These are organic compounds containing a sulfonamide group that is S-linked to a benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonamides |
|---|
| Direct Parent | Benzenesulfonamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzenesulfonamide
- Benzenesulfonyl group
- Pyrroline carboxylic acid or derivatives
- N-acyl urea
- Ureide
- Sulfonylurea
- Dicarboximide
- Pyrroline
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Aminosulfonyl compound
- Sulfonyl
- Carbonic acid derivative
- Urea
- Azacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-03di-0012290000-966a50af984de12b0ba8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0udi-2629000000-29b987358cc85159c5b8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-052f-2930000000-37fde3d60af9b32825ca | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-03di-0012290000-966a50af984de12b0ba8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0udi-2629000000-29b987358cc85159c5b8 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-03di-0002190000-0aaefa2e07605da97f81 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0udi-0409000000-91b493608ac508427593 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-004i-3911000000-78c0f40c965f78bfa591 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f6x-0609600000-78d1eafeec0253910006 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-1901000000-b9852a723bfd8ea4ca66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02vi-7900000000-f9c7acb1abe1893597e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0h2r-1908700000-4fe360933ffe931da22b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fmi-1927000000-d9a3ae76a7506ed0cee4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0229-4900000000-dcfccdc01980e9b7b49e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00222 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Glimepiride |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 5383 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|