| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:28:13 UTC |
|---|
| Update Date | 2016-11-09 01:15:37 UTC |
|---|
| Accession Number | CHEM017572 |
|---|
| Identification |
|---|
| Common Name | Fluocinolone acetonide |
|---|
| Class | Small Molecule |
|---|
| Description | Fluocinolone acetonide, with the formula 6-alpha, 9-alpha-difluoro-16-alpha, 17 alpha-acetonide, is a corticosteroid that presents a high lipophilicity. It has been used extensively in dermatological preparations and it has also been investigated thoroughly for its use in implantable corticosteroid devices. This type of device containing fluocinolone acetonide was developed by Taro Pharmaceuticals and approved by FDA in May 2016. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
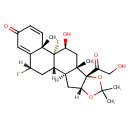
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6alpha,9alpha-Difluoro-16alpha-hydroxyprednisolone 16,17-acetonide | ChEBI | | 6alpha-Fluorotriamcinolone acetonide | ChEBI | | Acetonide de fluocinolone | ChEBI | | Acetonido de fluocinolona | ChEBI | | Coriphate | ChEBI | | Cortiplastol | ChEBI | | Derma-smoothe/fs | ChEBI | | Dermalar | ChEBI | | Flucinar | ChEBI | | Flucort | ChEBI | | Fluocet | ChEBI | | Fluocinolone 16,17-acetonide | ChEBI | | Fluocinoloni acetonidum | ChEBI | | Fluonid | ChEBI | | Fluotrex | ChEBI | | Fluovitif | ChEBI | | Flupollon | ChEBI | | Fluzon | ChEBI | | Iluvien | ChEBI | | Jellin | ChEBI | | Localyn | ChEBI | | Omniderm | ChEBI | | Percutina | ChEBI | | Prodermin | ChEBI | | Radiocin | ChEBI | | Retisert | ChEBI | | Sinalar | ChEBI | | Synalar | ChEBI | | Synamol | ChEBI | | Synandone | ChEBI | | Synandrone | ChEBI | | Synemol | ChEBI | | Synotic | ChEBI | | Synsac | ChEBI | | Tefunote | ChEBI | | 6a,9a-Difluoro-16a-hydroxyprednisolone 16,17-acetonide | Generator | | 6Α,9α-difluoro-16α-hydroxyprednisolone 16,17-acetonide | Generator | | 6a-Fluorotriamcinolone acetonide | Generator | | 6Α-fluorotriamcinolone acetonide | Generator | | Coriphic acid | Generator | | Flucinolone | HMDB | | Fluocinolonacetonidum | HMDB | | Acetonide, fluocinolone | HMDB | | Allergan brand OF fluocinolone acetonide | HMDB | | CO-Fluocin | HMDB | | FS, Derma-smooth | HMDB | | Fluortriamcinolone acetonide | HMDB | | Flurosyn | HMDB | | Galderma brand OF fluocinolone acetonide | HMDB | | Medicis brand 2 OF fluocinolone acetonide | HMDB | | Synalar HP | HMDB | | Acetonide, fluortriamcinolone | HMDB | | Alvadermo | HMDB | | Capex | HMDB | | Derma smooth FS | HMDB | | Fluocid | HMDB | | Geni brand OF fluocinolone acetonide | HMDB | | Grünenthal brand OF fluocinolone acetonide | HMDB | | Hill brand OF fluocinolone acetonide | HMDB | | Rugby brand OF fluocinolone acetonide | HMDB | | Savage brand OF fluocinolone acetonide | HMDB | | Septa brand OF fluocinolone acetonide | HMDB | | Bioglan brand OF fluocinolone acetonide | HMDB | | Centrum brand OF fluocinolone acetonide | HMDB | | Co fluocin | HMDB | | Derma-smooth FS | HMDB | | Fluodermo | HMDB | | Gelidina | HMDB | | Inkeysa brand OF fluocinolone acetonide | HMDB | | Jellisoft | HMDB | | Medicis brand 1 OF fluocinolone acetonide | HMDB | | Smaller brand OF fluocinolone acetonide | HMDB | | Synalar-HP | HMDB | | Syntex brand OF fluocinolone acetonide | HMDB | | Yamanouchi brand OF fluocinolone acetonide | HMDB | | Medphano brand OF fluocinolone acetonide | HMDB | | Cortiespec | HMDB | | Flusolgen | HMDB | | Inexfa brand OF fluocinolone acetonide | HMDB |
|
|---|
| Chemical Formula | C24H30F2O6 |
|---|
| Average Molecular Mass | 452.488 g/mol |
|---|
| Monoisotopic Mass | 452.201 g/mol |
|---|
| CAS Registry Number | 67-73-2 |
|---|
| IUPAC Name | (1S,2S,4R,8S,9S,11S,12R,13S,19S)-12,19-difluoro-11-hydroxy-8-(2-hydroxyacetyl)-6,6,9,13-tetramethyl-5,7-dioxapentacyclo[10.8.0.0²,⁹.0⁴,⁸.0¹³,¹⁸]icosa-14,17-dien-16-one |
|---|
| Traditional Name | capex |
|---|
| SMILES | [H][C@@]12C[C@@]3([H])[C@]4([H])C[C@]([H])(F)C5=CC(=O)C=C[C@]5(C)[C@@]4(F)[C@@H](O)C[C@]3(C)[C@@]1(OC(C)(C)O2)C(=O)CO |
|---|
| InChI Identifier | InChI=1S/C24H30F2O6/c1-20(2)31-19-9-13-14-8-16(25)15-7-12(28)5-6-21(15,3)23(14,26)17(29)10-22(13,4)24(19,32-20)18(30)11-27/h5-7,13-14,16-17,19,27,29H,8-11H2,1-4H3/t13-,14-,16-,17-,19+,21-,22-,23-,24+/m0/s1 |
|---|
| InChI Key | FEBLZLNTKCEFIT-VSXGLTOVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- 20-oxosteroid
- Pregnane-skeleton
- 3-oxo-delta-1,4-steroid
- 3-oxosteroid
- 9-halo-steroid
- 6-halo-steroid
- Halo-steroid
- Oxosteroid
- 11-beta-hydroxysteroid
- 11-hydroxysteroid
- Delta-1,4-steroid
- Ketal
- Alpha-hydroxy ketone
- Cyclic alcohol
- Meta-dioxolane
- Cyclic ketone
- Secondary alcohol
- Fluorohydrin
- Halohydrin
- Ketone
- Organoheterocyclic compound
- Acetal
- Oxacycle
- Organofluoride
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Carbonyl group
- Primary alcohol
- Alkyl fluoride
- Alcohol
- Organohalogen compound
- Alkyl halide
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4u-2790300000-f726c5edd782f086d667 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-1312190000-3d4982ca2270e0fc9e78 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0il9-0496400000-d158ff1b8dbcf078b8ff | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-00di-3981000000-e34007db14fcd7363a09 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0il9-0496400000-d158ff1b8dbcf078b8ff | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00di-3981000000-e34007db14fcd7363a09 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udr-0001900000-1b554128715f86bd3c32 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0k9l-2165900000-237d73e29007e691d6d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0c29-1091100000-6ed7a376ae3c8b2fd2df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1004900000-628e9b1a1feff59435e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kal-2004900000-7ffae0e5a19cb29f3732 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-6219000000-0de5fc8aacf929dedc54 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00591 |
|---|
| HMDB ID | HMDB0014729 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Fluocinolone_acetonide |
|---|
| Chemspider ID | 5980 |
|---|
| ChEBI ID | 31623 |
|---|
| PubChem Compound ID | 6215 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Jaffe GJ, Yang CH, Guo H, Denny JP, Lima C, Ashton P: Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci. 2000 Oct;41(11):3569-75. | | 2. Brumm MV, Nguyen QD: Fluocinolone acetonide intravitreal sustained release device--a new addition to the armamentarium of uveitic management. Int J Nanomedicine. 2007;2(1):55-64. | | 3. Goldstein DA, Godfrey DG, Hall A, Callanan DG, Jaffe GJ, Pearson PA, Usner DW, Comstock TL: Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol. 2007 Nov;125(11):1478-85. Epub 2007 Oct 8. | | 4. Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. | | 5. Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. | | 6. Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. | | 7. Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. | | 8. The lipid handbook with CD-ROM | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=21238799 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=21277557 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=21459216 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=23321234 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=23323586 |
|
|---|