| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:27:57 UTC |
|---|
| Update Date | 2016-11-09 01:15:37 UTC |
|---|
| Accession Number | CHEM017564 |
|---|
| Identification |
|---|
| Common Name | Artemisinin |
|---|
| Class | Small Molecule |
|---|
| Description | A sesquiterpene lactone obtained from sweet wormwood, Artemisia annua, which is used as an antimalarial for the treatment of multi-drug resistant strains of falciparum malaria. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
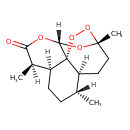
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,5,9-Trimethyl-(1R,4S,5R,9R,12S,13R)-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-one | ChEBI | | Arteannuin | ChEBI | | Artemisinina | ChEBI | | Artemisinine | ChEBI | | Artemisininum | ChEBI | | Huanghuahaosu | ChEBI | | Octahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano(4,3-J)-1,2-benzodioxepin-10(3H)-one | ChEBI | | QHS | ChEBI | | Qing hau sau | ChEBI | | Qinghaosu | ChEBI | | Quing hau sau | ChEBI | | Quinghaosu | MeSH | | Artemisinin | ChEBI |
|
|---|
| Chemical Formula | C15H22O5 |
|---|
| Average Molecular Mass | 282.336 g/mol |
|---|
| Monoisotopic Mass | 282.147 g/mol |
|---|
| CAS Registry Number | 63968-64-9 |
|---|
| IUPAC Name | (1R,4S,5R,8S,9R,12S,13R)-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.0⁴,¹³.0⁸,¹³]hexadecan-10-one |
|---|
| Traditional Name | (+)-artemisinin |
|---|
| SMILES | [H][C@@]1(C)CC[C@@]2([H])[C@@]([H])(C)C(=O)O[C@]3([H])O[C@@]4(C)CC[C@]1([H])[C@@]23OO4 |
|---|
| InChI Identifier | InChI=1S/C15H22O5/c1-8-4-5-11-9(2)12(16)17-13-15(11)10(8)6-7-14(3,18-13)19-20-15/h8-11,13H,4-7H2,1-3H3/t8-,9-,10+,11+,13-,14-,15-/m1/s1 |
|---|
| InChI Key | BLUAFEHZUWYNDE-NNWCWBAJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as terpene lactones. These are prenol lipids containing a lactone ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene lactones |
|---|
| Direct Parent | Terpene lactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Artemisinin skeleton
- Terpene lactone
- Sesquiterpenoid
- Delta valerolactone
- Delta_valerolactone
- Oxepane
- Oxane
- 1,2,4-trioxane
- Carboxylic acid ester
- Lactone
- Dialkyl peroxide
- Monocarboxylic acid or derivatives
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0007-0901000000-4fa10fe72dc24b85ac9c | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-014i-0190000000-3391c971e951545100ee | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0ldj-0960000000-ff3a9e18db5c8c53bcb4 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-01wb-0910000000-42c2b516c8462b3dd492 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-01x1-0900000000-5c3e44b5d52be3adbba2 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0a7l-0900000000-01583256dc6d13c1315f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0a59-0900000000-189290ae52e9283563ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-81624c4cf74214fdaa9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1090000000-280b3ca0132072b52506 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9100000000-73e8efda73cc4b76c149 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001r-0090000000-f9b1c888e060cd130d8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0019-1090000000-53e88c0fa4a2405b229c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-4690000000-687f2a3a4ca745522896 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13132 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003359 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-7561 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Artemisinin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 223316 |
|---|
| PubChem Compound ID | 68827 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|