| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:27:51 UTC |
|---|
| Update Date | 2016-11-09 01:15:37 UTC |
|---|
| Accession Number | CHEM017560 |
|---|
| Identification |
|---|
| Common Name | (6R)-Tetrahydrobiopterin dihydrochloride |
|---|
| Class | Small Molecule |
|---|
| Description | The dihydrochloride salt of sapropterin. It is used for the diagnosis and treatment of variant forms of phenylketonuria (hyperphenylalaninaemia) associated with tetrahydrobiopterin deficiency. Natural cofactor for phenylalanine hydroxylase, tyrosine hydroxylase, tryptophan hydroxylase, and nitric oxide synthetase. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
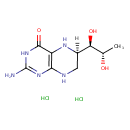
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (6R)-Tetrahydrobiopterin dihydrochloride | ChEBI | | (6R)-Tetrahydrobiopterin hydrochloride | ChEBI | | Sapropterin 2HCL | ChEBI | | Sapropterin hydrochloride | ChEBI | | Biopten | Kegg | | Kuvan | Kegg | | 5,6,7,8-Tetrahydrobiopterin | MeSH | | tetrahydro-6-Biopterin | MeSH | | Sapropterin dihydrochloride | MeSH | | Tetrahydrobiopterin | MeSH | | 5,6,7,8-tetrahydro-L-Erythrobiopterin | MeSH | | 5,6,7,8-erythro-Tetrahydrobiopterin | MeSH | | 5,6,7,8-Tetrahydrobiopterin, (S-(r*,s*))-isomer | MeSH | | 5,6,7,8-Tetrahydrodictyopterin | MeSH | | BPH4 | MeSH | | D-threo-Tetrahydrobiopterin | MeSH | | 6R-L-erythro-5,6,7,8-Tetrahydrobiopterin | MeSH | | THBP | MeSH | | 6R-BH4 | MeSH | | Phenylalanine hydroxylase cofactor | MeSH | | Sapropterin | MeSH |
|
|---|
| Chemical Formula | C9H17Cl2N5O3 |
|---|
| Average Molecular Mass | 314.170 g/mol |
|---|
| Monoisotopic Mass | 313.071 g/mol |
|---|
| CAS Registry Number | 69056-38-8 |
|---|
| IUPAC Name | (6R)-2-amino-6-[(1R,2S)-1,2-dihydroxypropyl]-3,4,5,6,7,8-hexahydropteridin-4-one dihydrochloride |
|---|
| Traditional Name | 6R-5,6,7,8-tetrahydrobiopterin dihydrochloride |
|---|
| SMILES | Cl.Cl.[H][C@@]1(CNC2=C(N1)C(=O)NC(N)=N2)[C@@H](O)[C@H](C)O |
|---|
| InChI Identifier | InChI=1S/C9H15N5O3.2ClH/c1-3(15)6(16)4-2-11-7-5(12-4)8(17)14-9(10)13-7;;/h3-4,6,12,15-16H,2H2,1H3,(H4,10,11,13,14,17);2*1H/t3-,4+,6-;;/m0../s1 |
|---|
| InChI Key | RKSUYBCOVNCALL-NTVURLEBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Biopterins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biopterin
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Pyrimidine
- 1,3-aminoalcohol
- Heteroaromatic compound
- Vinylogous amide
- 1,2-aminoalcohol
- 1,2-diol
- Secondary alcohol
- Azacycle
- Secondary amine
- Organic nitrogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Amine
- Alcohol
- Organopnictogen compound
- Hydrochloride
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-1f734a32f72840dcbe52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0009000000-1f734a32f72840dcbe52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0009000000-1f734a32f72840dcbe52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-94e05be52826768dc03a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0009000000-94e05be52826768dc03a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0009000000-94e05be52826768dc03a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT001133 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tetrahydrobiopterin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 32120 |
|---|
| PubChem Compound ID | 636369 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|