| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:27:22 UTC |
|---|
| Update Date | 2016-11-09 01:15:37 UTC |
|---|
| Accession Number | CHEM017549 |
|---|
| Identification |
|---|
| Common Name | Kainic acid |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
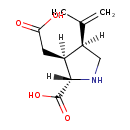
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S-(2alpha,3beta,4beta))-2-Carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid | ChEBI | | Acide kainique | ChEBI | | Acido kainico | ChEBI | | Acidum kainicum | ChEBI | | alpha- Kainic acid | ChEBI | | alpha-Kainic acid | ChEBI | | Digenic acid | ChEBI | | Digenin | ChEBI | | Digensaeure | ChEBI | | Helminal | ChEBI | | Kainsaeure | ChEBI | | L-alpha-Kainic acid | ChEBI | | Kainate | Kegg | | (2S-(2a,3b,4b))-2-Carboxy-4-(1-methylethenyl)-3-pyrrolidineacetate | Generator | | (2S-(2a,3b,4b))-2-Carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid | Generator | | (2S-(2alpha,3beta,4beta))-2-Carboxy-4-(1-methylethenyl)-3-pyrrolidineacetate | Generator | | (2S-(2Α,3β,4β))-2-carboxy-4-(1-methylethenyl)-3-pyrrolidineacetate | Generator | | (2S-(2Α,3β,4β))-2-carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid | Generator | | a- Kainate | Generator | | a- Kainic acid | Generator | | alpha- Kainate | Generator | | Α- kainate | Generator | | Α- kainic acid | Generator | | a-Kainate | Generator | | a-Kainic acid | Generator | | alpha-Kainate | Generator | | Α-kainate | Generator | | Α-kainic acid | Generator | | Digenate | Generator | | L-a-Kainate | Generator | | L-a-Kainic acid | Generator | | L-alpha-Kainate | Generator | | L-Α-kainate | Generator | | L-Α-kainic acid | Generator | | Acid, digenic | MeSH | | Acid, kainic | MeSH |
|
|---|
| Chemical Formula | C10H15NO4 |
|---|
| Average Molecular Mass | 213.230 g/mol |
|---|
| Monoisotopic Mass | 213.100 g/mol |
|---|
| CAS Registry Number | 487-79-6 |
|---|
| IUPAC Name | (2S,3S,4S)-3-(carboxymethyl)-4-(prop-1-en-2-yl)pyrrolidine-2-carboxylic acid |
|---|
| Traditional Name | kainic acid |
|---|
| SMILES | [H][C@@]1(CN[C@]([H])(C(O)=O)[C@@]1([H])CC(O)=O)C(C)=C |
|---|
| InChI Identifier | InChI=1S/C10H15NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h6-7,9,11H,1,3-4H2,2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 |
|---|
| InChI Key | VLSMHEGGTFMBBZ-OOZYFLPDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as kainoids. These are non-proteigenous amino acids with a structure characterized by the presence of a pyrrolidine ring bearing two dicarboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Kainoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Kainoid skeleton
- Proline or derivatives
- Alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- Dicarboxylic acid or derivatives
- Pyrrolidine
- Amino acid
- Carboxylic acid
- Secondary aliphatic amine
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Amine
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0j4j-0920000000-15f00fdd361fff0dfe48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gi1-1900000000-348beb87de321725e4dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00yi-9800000000-6c7ccd63ebd73b654dfe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03xr-0960000000-4daaae83398a853f1fac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-0920000000-2c0f6c826035120f23af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0609-7900000000-aef12c986f9fa93935b8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Kainic acid |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 31746 |
|---|
| PubChem Compound ID | 10255 |
|---|
| Kegg Compound ID | C12819 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|