| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:27:10 UTC |
|---|
| Update Date | 2016-11-09 01:15:37 UTC |
|---|
| Accession Number | CHEM017541 |
|---|
| Identification |
|---|
| Common Name | Metaflumizone |
|---|
| Class | Small Molecule |
|---|
| Description | Metaflumizone is a semicarbazone insecticide indicated for the veterinary treatment of fleas and ticks, marketed under the brand name ProMeris.
A discontinued variant of ProMeris, called ProMeris Duo or Promeris for Dogs, was indicated for canine use and was a formulated blend of metaflumizone and amitraz. The metaflumizone-only formulation is waterproof and typically remain effective for 30–45 days in a cutaneous application at the base of the neck. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
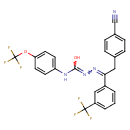
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Metaflumizone | MeSH | | N'-{[2-(4-cyanophenyl)-1-[3-(trifluoromethyl)phenyl]ethylidene]amino}-N-[4-(trifluoromethoxy)phenyl]carbamimidate | Generator |
|
|---|
| Chemical Formula | C24H16F6N4O2 |
|---|
| Average Molecular Mass | 506.408 g/mol |
|---|
| Monoisotopic Mass | 506.118 g/mol |
|---|
| CAS Registry Number | 139968-49-3 |
|---|
| IUPAC Name | N'-{[2-(4-cyanophenyl)-1-[3-(trifluoromethyl)phenyl]ethylidene]amino}-N-[4-(trifluoromethoxy)phenyl]carbamimidic acid |
|---|
| Traditional Name | N'-{[2-(4-cyanophenyl)-1-[3-(trifluoromethyl)phenyl]ethylidene]amino}-N-[4-(trifluoromethoxy)phenyl]carbamimidic acid |
|---|
| SMILES | OC(NC1=CC=C(OC(F)(F)F)C=C1)=NN=C(CC1=CC=C(C=C1)C#N)C1=CC(=CC=C1)C(F)(F)F |
|---|
| InChI Identifier | InChI=1S/C24H16F6N4O2/c25-23(26,27)18-3-1-2-17(13-18)21(12-15-4-6-16(14-31)7-5-15)33-34-22(35)32-19-8-10-20(11-9-19)36-24(28,29)30/h1-11,13H,12H2,(H2,32,34,35) |
|---|
| InChI Key | MIFOMMKAVSCNKQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- N-phenylurea
- Trifluoromethylbenzene
- Phenoxy compound
- Benzonitrile
- Phenol ether
- Monocyclic benzene moiety
- Semicarbazone
- Benzenoid
- Semicarbazide
- Carbonic acid derivative
- Trihalomethane
- Nitrile
- Carbonitrile
- Alkyl halide
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Alkyl fluoride
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Halomethane
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zfr-0159370000-4af94b2521dc56bfa0b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05i0-0984440000-ab7beb34f809a19492ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-0944000000-b13ba9684045122826aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0kdi-0379150000-a7b030744c6fa4c7a8f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0039000000-72cfa243bc2892f06c75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pdi-2941000000-7b0c57ee8b8a23ba147d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000090000-0dcee4eb3be5ce19da94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0090010000-fdb1011f39763f8ba035 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0l90-2491000000-6e4591f307ef6385ae9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fk9-0098000000-ce0d00b9dbaba7df4b61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uk9-0296200000-1b1fca59f86b46ee984f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uk9-0498000000-74769a1c73309dc6fcbc | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Metaflumizone |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 20056430 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|