| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:26:38 UTC |
|---|
| Update Date | 2016-11-09 01:15:37 UTC |
|---|
| Accession Number | CHEM017534 |
|---|
| Identification |
|---|
| Common Name | Sulindac sulfone |
|---|
| Class | Small Molecule |
|---|
| Description | A sulfone metabolite of sulindac that inhibits cell growth by inducing apoptosis independently of cyclooxygenase inhibition. It inhibits the development and induces regression of premalignant adenomatous polyps. Lipoxygenase and Cox-2 inhibitor. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
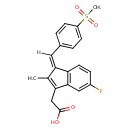
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-Fluoro-2-methyl-1-((Z)-p-(methylsulfonyl)benzylidene)indene-3-acetic acid | ChEBI | | cis-5-Fluoro-2-methyl-1-(p-methylsulfonylbenzylidenyl)indene-3-acetic acid | ChEBI | | Exisulind | ChEBI | | 5-Fluoro-2-methyl-1-((Z)-p-(methylsulfonyl)benzylidene)indene-3-acetate | Generator | | 5-Fluoro-2-methyl-1-((Z)-p-(methylsulphonyl)benzylidene)indene-3-acetate | Generator | | 5-Fluoro-2-methyl-1-((Z)-p-(methylsulphonyl)benzylidene)indene-3-acetic acid | Generator | | cis-5-Fluoro-2-methyl-1-(p-methylsulfonylbenzylidenyl)indene-3-acetate | Generator | | cis-5-Fluoro-2-methyl-1-(p-methylsulphonylbenzylidenyl)indene-3-acetate | Generator | | cis-5-Fluoro-2-methyl-1-(p-methylsulphonylbenzylidenyl)indene-3-acetic acid | Generator | | Sulindac sulphone | Generator | | (5-Fluoro-2-methyl-1-(3,4,5-trimethoxybenzylidene)-3-(N-benzyl)-indene)-acetamide | MeSH | | FGN-1 | MeSH | | Aptosyn | MeSH | | Sulindac sulfone, (Z)-isomer | MeSH | | 2-[(3Z)-6-fluoro-2-Methyl-3-[(4-methylsulfonylphenyl)methylidene]inden-1-yl]acetate | Generator | | 2-[(3Z)-6-fluoro-2-Methyl-3-[(4-methylsulphonylphenyl)methylidene]inden-1-yl]acetate | Generator | | 2-[(3Z)-6-fluoro-2-Methyl-3-[(4-methylsulphonylphenyl)methylidene]inden-1-yl]acetic acid | Generator | | Sulindac sulfone | MeSH |
|
|---|
| Chemical Formula | C20H17FO4S |
|---|
| Average Molecular Mass | 372.410 g/mol |
|---|
| Monoisotopic Mass | 372.083 g/mol |
|---|
| CAS Registry Number | 59973-80-7 |
|---|
| IUPAC Name | 2-[(1Z)-5-fluoro-1-[(4-methanesulfonylphenyl)methylidene]-2-methyl-1H-inden-3-yl]acetic acid |
|---|
| Traditional Name | sulindac sulfone |
|---|
| SMILES | [H]\C(=C1/C(C)=C(CC(O)=O)C2=C1C=CC(F)=C2)C1=CC=C(C=C1)S(C)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C20H17FO4S/c1-12-17(9-13-3-6-15(7-4-13)26(2,24)25)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- |
|---|
| InChI Key | MVGSNCBCUWPVDA-MFOYZWKCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indenes and isoindenes. Indenes and isoindenes are compounds containing an indene moiety(which consists of a cyclopentadiene fused to a benzene ring), or a isoindene moiety (which consists of a cyclopentadiene fused to cyclohexadiene ring). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Indenes and isoindenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Indenes and isoindenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzenesulfonyl group
- Indene
- Aryl fluoride
- Aryl halide
- Monocyclic benzene moiety
- Sulfonyl
- Sulfone
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Organohalogen compound
- Organofluoride
- Organooxygen compound
- Organosulfur compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0009000000-c2351a6577cc7d71d48b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0139000000-bfbff4e9d46df020adea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-3393000000-160aaaa293cfde04b13a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00fr-0009000000-48ae194abe05841cd6e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00b9-4019000000-ed831a5d0006b728154a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9010000000-d144d4b5d5ebd561143d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Exisulind |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 64212 |
|---|
| PubChem Compound ID | 5472495 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|