| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:26:07 UTC |
|---|
| Update Date | 2016-11-09 01:15:36 UTC |
|---|
| Accession Number | CHEM017527 |
|---|
| Identification |
|---|
| Common Name | Yohimbine |
|---|
| Class | Small Molecule |
|---|
| Description | A plant alkaloid with alpha-2-adrenergic blocking activity. Yohimbine has been used as a mydriatic and in the treatment of impotence. It is also alleged to be an aphrodisiac. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
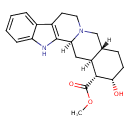
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Yohimbine | ChEBI | | (16alpha,17alpha)-17-Hydroxyyohimban-16-carboxylic acid methyl ester | ChEBI | | 17alpha-Hydroxyyohimban-16alpha-carboxylic acid methyl ester | ChEBI | | Aphrodine | ChEBI | | Corynine | ChEBI | | Johimbin | ChEBI | | Quebrachin | ChEBI | | Quebrachine | ChEBI | | Yohimbic acid methyl ester | ChEBI | | Yohimbin | ChEBI | | (16a,17a)-17-Hydroxyyohimban-16-carboxylate methyl ester | Generator | | (16a,17a)-17-Hydroxyyohimban-16-carboxylic acid methyl ester | Generator | | (16alpha,17alpha)-17-Hydroxyyohimban-16-carboxylate methyl ester | Generator | | (16Α,17α)-17-hydroxyyohimban-16-carboxylate methyl ester | Generator | | (16Α,17α)-17-hydroxyyohimban-16-carboxylic acid methyl ester | Generator | | 17a-Hydroxyyohimban-16a-carboxylate methyl ester | Generator | | 17a-Hydroxyyohimban-16a-carboxylic acid methyl ester | Generator | | 17alpha-Hydroxyyohimban-16alpha-carboxylate methyl ester | Generator | | 17Α-hydroxyyohimban-16α-carboxylate methyl ester | Generator | | 17Α-hydroxyyohimban-16α-carboxylic acid methyl ester | Generator | | Yohimbate methyl ester | Generator | | Aphrodyne | HMDB | | Aventis brand OF yohimbine hydrochloride | HMDB | | Solvay brand OF yohimbine hydrochloride | HMDB | | Yohimbine hydrochloride | HMDB | | Aphrodine hydrochloride | HMDB | | Hydrochloride, yohimbine | HMDB | | Pluriviron | HMDB | | Rauwolscine | HMDB | | Palisades brand OF yohimbine hydrochloride | HMDB | | Star brand OF yohimbine hydrochloride | HMDB | | StegroPharm brand OF yohimbine hydrochloride | HMDB | | Tartrate, corynanthine | HMDB | | Corynanthine | HMDB | | Corynanthine tartrate | HMDB | | Glenwood brand OF yohimbine hydrochloride | HMDB | | Hydrochloride, aphrodine | HMDB | | Kramer brand OF yohimbine hydrochloride | HMDB | | Rauhimbine | HMDB | | Yocon | HMDB | | Yohimbin spiegel | HMDB | | Yohimbine houdé | HMDB | | Yohimex | HMDB |
|
|---|
| Chemical Formula | C21H26N2O3 |
|---|
| Average Molecular Mass | 354.443 g/mol |
|---|
| Monoisotopic Mass | 354.194 g/mol |
|---|
| CAS Registry Number | 146-48-5 |
|---|
| IUPAC Name | methyl (1S,15R,18S,19R,20S)-18-hydroxy-3,13-diazapentacyclo[11.8.0.0²,¹⁰.0⁴,⁹.0¹⁵,²⁰]henicosa-2(10),4,6,8-tetraene-19-carboxylate |
|---|
| Traditional Name | yohimbine |
|---|
| SMILES | [H][C@@]12CC[C@H](O)[C@H](C(=O)OC)[C@@]1([H])C[C@]1([H])N(CCC3=C1NC1=CC=CC=C31)C2 |
|---|
| InChI Identifier | InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 |
|---|
| InChI Key | BLGXFZZNTVWLAY-SCYLSFHTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as yohimbine alkaloids. These are alkaloids containing the pentacyclic yohimban skeleton. The Yohimbinoid alkaloids contain a carbocyclic ring E arising through C-17 to C-18 bond formation in a corynantheine precursor. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Yohimbine alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Yohimbine alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Yohimbine
- Corynanthean skeleton
- Yohimbine alkaloid
- Beta-carboline
- Pyridoindole
- 3-alkylindole
- Indole
- Indole or derivatives
- Aralkylamine
- Beta-hydroxy acid
- Hydroxy acid
- Benzenoid
- Piperidine
- Heteroaromatic compound
- Methyl ester
- Cyclic alcohol
- Pyrrole
- Amino acid or derivatives
- Tertiary aliphatic amine
- Carboxylic acid ester
- Tertiary amine
- Secondary alcohol
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organoheterocyclic compound
- Organic oxide
- Organonitrogen compound
- Organic nitrogen compound
- Organooxygen compound
- Amine
- Carbonyl group
- Organic oxygen compound
- Alcohol
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | - methyl 17-hydroxy-20xi-yohimban-16-carboxylate (CHEBI:10093 )
- Indole alkaloids (C09256 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-008i-0396000000-43448cca1e1ad4343ac0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00xs-2139200000-8288a21cfcdae82fc694 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-3921000000-e28dd8692e2b8dd63330 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0019000000-98fd50566f845231fbed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-0129000000-49a3b77851cb2f327e51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ffx-0891000000-1297a98bf2d980f32361 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-6448f2e7be3b7fa35128 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0009000000-a436855e9ddc5d8e061e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07ef-3956000000-386e3fa9fb16f1a30cf6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0009000000-b3d998cb3c47c9700660 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0009000000-42b2cee0514145bdbc16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002g-0695000000-7eacdb4b3fbff0521dcd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-1bc1d16b561be98b6dbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0095000000-d9723526ae361fda41c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f96-0192000000-a13fb9eefdc725558673 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01392 |
|---|
| HMDB ID | HMDB0015464 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001789 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Yohimbine |
|---|
| Chemspider ID | 8622 |
|---|
| ChEBI ID | 10093 |
|---|
| PubChem Compound ID | 8969 |
|---|
| Kegg Compound ID | C09256 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|