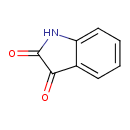
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014j-3900000000-cfbe2c69370cb821719e | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0002-0900000000-b7740f917ea3a513cccf | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-014i-0900000000-7cc1e59d64b0f9fe75c7 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9200000000-7222eca5f15462cb52d6 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9000000000-9d8ae3e3d93ebbae650e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9000000000-5f853d2ccc4bbe2b3a89 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0002-0900000000-4d14e90a2ccd915ec028 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0002-1900000000-efd2670c6d91416ca123 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-001l-3900000000-a768411b3bdce1e8bbf7 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0f89-3900000000-00d17037a416b9858a30 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0fc0-7900000000-b2e65fbe547a142b1b3e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-001j-0900000000-6129e468197154787423 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-01ot-6900000000-e172235ae1d7d5980b78 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0g5d-9600000000-3abf3b0049a56410c636 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-014l-9100000000-0fb3353ec5204cb4b15c | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0ik9-9000000000-82722063406d135a28ca | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0fc0-7900000000-b2e65fbe547a142b1b3e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0002-1900000000-efd2670c6d91416ca123 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-4d14e90a2ccd915ec028 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-001l-3900000000-a768411b3bdce1e8bbf7 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-e31d27d5029a58203e08 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-a71d92faa742d62f15dc | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kg-9200000000-4c49ea126a926312a044 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-664b6d8a1a57af39e032 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-1900000000-4fdb6fbe94e7299a7a8a | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-e0ec72a316f86a8a4615 | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |