| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:16:32 UTC |
|---|
| Update Date | 2016-11-09 01:15:34 UTC |
|---|
| Accession Number | CHEM017362 |
|---|
| Identification |
|---|
| Common Name | 2,2-Dimethylbutanoic acid |
|---|
| Class | Small Molecule |
|---|
| Description | A branched-chain fatty acid and metabolite of the lactone prodrug simvastatin, whose sodium salt is potentially useful for the treatment of thalassaemias and haemoglobinopathies. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
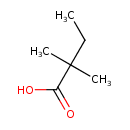
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha,alpha-Dimethylbutanoic acid | ChEBI | | alpha,alpha-Dimethylbutyric acid | ChEBI | | DMB | ChEBI | | a,a-Dimethylbutanoate | Generator | | a,a-Dimethylbutanoic acid | Generator | | alpha,alpha-Dimethylbutanoate | Generator | | Α,α-dimethylbutanoate | Generator | | Α,α-dimethylbutanoic acid | Generator | | a,a-Dimethylbutyrate | Generator | | a,a-Dimethylbutyric acid | Generator | | alpha,alpha-Dimethylbutyrate | Generator | | Α,α-dimethylbutyrate | Generator | | Α,α-dimethylbutyric acid | Generator | | 2,2-Dimethylbutyrate | Generator | | 2,2-Dimethylbutanoate | Generator | | Sodium 2,2-dimethylbutyrate | MeSH | | 2,2-Dimethylbutyric acid | MeSH | | ST-20 Compound | MeSH | | 2,2-DMB | MeSH | | 2,2-Dimethylbutanoic acid | MeSH | | SDMB | MeSH |
|
|---|
| Chemical Formula | C6H12O2 |
|---|
| Average Molecular Mass | 116.160 g/mol |
|---|
| Monoisotopic Mass | 116.084 g/mol |
|---|
| CAS Registry Number | 595-37-9 |
|---|
| IUPAC Name | 2,2-dimethylbutanoic acid |
|---|
| Traditional Name | 2,2-dimethyl butyric acid |
|---|
| SMILES | CCC(C)(C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H12O2/c1-4-6(2,3)5(7)8/h4H2,1-3H3,(H,7,8) |
|---|
| InChI Key | VUAXHMVRKOTJKP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methyl-branched fatty acids. These are fatty acids with an acyl chain that has a methyl branch. Usually, they are saturated and contain only one or more methyl group. However, branches other than methyl may be present. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Methyl-branched fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methyl-branched fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00bc-9100000000-322b8ae9e48656cf43b7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-8900000000-6414401c45360d4b4d3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00xr-9100000000-457ce6605eef7df7761a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-9000000000-a4a0d65d76c617b30bc2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1900000000-dbeed058312961eeb960 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00xr-9600000000-b3949ee905d2924dc674 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-060c-9000000000-b54bffb1b6686f609c1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-9100000000-4dbb11d9b5fe6d97cc9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fu-9000000000-ccf2e72c042db4d4433f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9000000000-4e1b57522a4141d30963 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-f02a8c6af96b787bcd37 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-0e94be25ed9046c8caf8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-d948d5d95ae14e701f57 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0245333 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 11193 |
|---|
| ChEBI ID | 38649 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|