| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:16:11 UTC |
|---|
| Update Date | 2016-11-09 01:15:34 UTC |
|---|
| Accession Number | CHEM017356 |
|---|
| Identification |
|---|
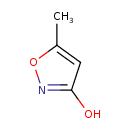
| Common Name | Hymexazol |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of isoxazoles carrying hydroxy and methyl substituents at positions 3 and 5 respectively. It is used worldwide as a systemic soil and seed fungicide for the control of diseases caused by Fusarium, Aphanomyces, Pythium, and Corticium spp. in rice, sugarbeet, fodderbeet, vegetables, cucurbits, and ornamentals. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Hydroxy-5-methylisoxazole | ChEBI | | 5-Methyl-3(2H)-isoxazolone | ChEBI | | 5-Methyl-3-hydroxyisoxazole | ChEBI | | 5-Methylisoxazol-3-ol | ChEBI | | F 319 | ChEBI | | F-319 | ChEBI | | Hydroxyisoxazole | ChEBI | | Hymexazole | ChEBI | | NSC 217971 | ChEBI | | SF-6505 | ChEBI | | Bucide calcium | MeSH | | Bucide monosodium | MeSH | | Bucide potassium | MeSH | | Bucide | MeSH |
|
|---|
| Chemical Formula | C4H5NO2 |
|---|
| Average Molecular Mass | 99.089 g/mol |
|---|
| Monoisotopic Mass | 99.032 g/mol |
|---|
| CAS Registry Number | 10004-44-1 |
|---|
| IUPAC Name | 5-methyl-1,2-oxazol-3-ol |
|---|
| Traditional Name | 3-hydroxy-5-methylisoxazole |
|---|
| SMILES | CC1=CC(O)=NO1 |
|---|
| InChI Identifier | InChI=1S/C4H5NO2/c1-3-2-4(6)5-7-3/h2H,1H3,(H,5,6) |
|---|
| InChI Key | KGVPNLBXJKTABS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isoxazoles. These are heterocyclic organic compounds containing an isoxazole moiety, with a structure characterized by a five-member aromatic ring with one oxygen atom and one nitrogen atom at ring positions 1 and 2, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Isoxazoles |
|---|
| Direct Parent | Isoxazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Heteroaromatic compound
- Isoxazole
- Lactam
- Oxacycle
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-1d84afd8443752800a27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-da4fddd614aa1baed07f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9000000000-865b204061e62cf1f856 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-cfdb2fdaf779a7a3d716 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9000000000-d217d1605abe41d584b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-9000000000-9f05a9afcd5a0119f14e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 5827 |
|---|
| PubChem Compound ID | 24781 |
|---|
| Kegg Compound ID | C11103 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|