| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:15:05 UTC |
|---|
| Update Date | 2016-11-09 01:15:34 UTC |
|---|
| Accession Number | CHEM017334 |
|---|
| Identification |
|---|
| Common Name | Scopolamine hydrobromide |
|---|
| Class | Small Molecule |
|---|
| Description | A hydrobromide that is obtained by reaction of scopolamine with hydrogen bromide. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
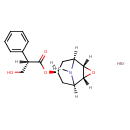
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Hyoscine hydrobromide | ChEBI | | (-)-Hyoscine hydrobromide (anhydrous) | ChEBI | | (-)-Scopolamine bromide | ChEBI | | (-)-Scopolamine bromide (anhydrous) | ChEBI | | (-)-Scopolamine hydrobromide | ChEBI | | (-)-Scopolamine hydrobromide (anhydrous) | ChEBI | | Hyoscine bromide | ChEBI | | Hyoscine bromide (anhydrous) | ChEBI | | Scopolamine bromide | ChEBI | | Scopolamine bromide (anhydrous) | ChEBI | | Scopolamine hydrobromide | ChEBI | | Scopolaminium bromide | ChEBI | | Scopolaminium bromide (anhydrous) | ChEBI | | Scopolammonium bromide | ChEBI | | Scopolammonium bromide (anhydrous) | ChEBI |
|
|---|
| Chemical Formula | C17H22BrNO4 |
|---|
| Average Molecular Mass | 384.270 g/mol |
|---|
| Monoisotopic Mass | 383.073 g/mol |
|---|
| CAS Registry Number | 114-49-8 |
|---|
| IUPAC Name | (1R,2R,4S,5S,7S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.0²,⁴]nonan-7-yl (2S)-3-hydroxy-2-phenylpropanoate hydrobromide |
|---|
| Traditional Name | (1R,2R,4S,5S,7S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.0²,⁴]nonan-7-yl (2S)-3-hydroxy-2-phenylpropanoate hydrobromide |
|---|
| SMILES | Br.[H][C@](CO)(C(=O)O[C@]1([H])C[C@@]2([H])N(C)[C@@]([H])(C1)[C@]1([H])O[C@]21[H])C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C17H21NO4.BrH/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10;/h2-6,11-16,19H,7-9H2,1H3;1H/t11-,12-,13-,14+,15-,16+;/m1./s1 |
|---|
| InChI Key | WTGQALLALWYDJH-MOUKNHLCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-hydroxy acid
- Monocyclic benzene moiety
- Morpholine
- Oxazinane
- Piperidine
- N-alkylpyrrolidine
- Benzenoid
- Pyrrolidine
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary amine
- Tertiary aliphatic amine
- Organoheterocyclic compound
- Oxacycle
- Azacycle
- Carboxylic acid derivative
- Dialkyl ether
- Oxirane
- Ether
- Monocarboxylic acid or derivatives
- Alcohol
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic oxide
- Amine
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Primary alcohol
- Hydrobromide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-f6dec275fffcf0f30a4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0009000000-f6dec275fffcf0f30a4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0009000000-f6dec275fffcf0f30a4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-f626f7e8f739a2192ec5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0009000000-f626f7e8f739a2192ec5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0009000000-f626f7e8f739a2192ec5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 61271 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|