| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 04:13:43 UTC |
|---|
| Update Date | 2016-11-09 01:15:34 UTC |
|---|
| Accession Number | CHEM017301 |
|---|
| Identification |
|---|
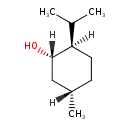
| Common Name | (1S,2R,5S)-(+)-Menthol |
|---|
| Class | Small Molecule |
|---|
| Description | A p-menthan-3-ol which has (1S,2R,5S)-stereochemistry. In contrast to (-)-menthol, the (+)-enantiomer occurs only rarely in nature. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-(1S,2R,5S)-Menthol | ChEBI | | (+)-(1S,3S,4R)-Menthol | ChEBI | | (1S,2R,5S)-(+)-Menthol | ChEBI | | (1S,2R,5S)-Menthol | ChEBI | | D-Menthol | ChEBI | | Menthol | MeSH | | Menthol, (1alpha,2beta,5alpha)-isomer | MeSH |
|
|---|
| Chemical Formula | C10H20O |
|---|
| Average Molecular Mass | 156.269 g/mol |
|---|
| Monoisotopic Mass | 156.151 g/mol |
|---|
| CAS Registry Number | 15356-60-2 |
|---|
| IUPAC Name | (1S,2R,5S)-5-methyl-2-(propan-2-yl)cyclohexan-1-ol |
|---|
| Traditional Name | l-menthol |
|---|
| SMILES | [H][C@]1(C)CC[C@]([H])(C(C)C)[C@@]([H])(O)C1 |
|---|
| InChI Identifier | InChI=1S/C10H20O/c1-7(2)9-5-4-8(3)6-10(9)11/h7-11H,4-6H2,1-3H3/t8-,9+,10-/m0/s1 |
|---|
| InChI Key | NOOLISFMXDJSKH-AEJSXWLSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Menthane monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-menthane monoterpenoid
- Monocyclic monoterpenoid
- Cyclohexanol
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0900000000-39d327015211bdc2b4e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-6900000000-67e6c41c2a8d759d6caf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-9200000000-88888989ba15a9dc0269 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0900000000-3a700dc611fb070e6b45 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0900000000-86ed17c709dc4636ad41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bti-6900000000-fc116bf41f068d3f6f75 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11344 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 76306 |
|---|
| PubChem Compound ID | 165675 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|