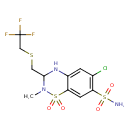| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:59:44 UTC |
|---|
| Update Date | 2016-11-09 01:15:32 UTC |
|---|
| Accession Number | CHEM017182 |
|---|
| Identification |
|---|
| Common Name | Polythiazide |
|---|
| Class | Small Molecule |
|---|
| Description | A thiazide diuretic with actions and uses similar to those of hydrochlorothiazide. (From Martindale, The Extra Pharmacopoeia, 30th ed, p826) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | |
|---|
| Chemical Formula | C11H13ClF3N3O4S3 |
|---|
| Average Molecular Mass | 439.882 g/mol |
|---|
| Monoisotopic Mass | 438.971 g/mol |
|---|
| CAS Registry Number | 346-18-9 |
|---|
| IUPAC Name | 6-chloro-2-methyl-1,1-dioxo-3-{[(2,2,2-trifluoroethyl)sulfanyl]methyl}-3,4-dihydro-2H-1λ⁶,2,4-benzothiadiazine-7-sulfonamide |
|---|
| Traditional Name | polythiazide |
|---|
| SMILES | CN1C(CSCC(F)(F)F)NC2=CC(Cl)=C(C=C2S1(=O)=O)S(N)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C11H13ClF3N3O4S3/c1-18-10(4-23-5-11(13,14)15)17-7-2-6(12)8(24(16,19)20)3-9(7)25(18,21)22/h2-3,10,17H,4-5H2,1H3,(H2,16,19,20) |
|---|
| InChI Key | CYLWJCABXYDINA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2,4-benzothiadiazine-1,1-dioxides. These are aromatic heterocyclic compounds containing a 1,2,4-benzothiadiazine ring system with two S=O bonds at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Thiadiazines |
|---|
| Sub Class | Benzothiadiazines |
|---|
| Direct Parent | 1,2,4-benzothiadiazine-1,1-dioxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2,4-benzothiadiazine-1,1-dioxide
- Secondary aliphatic/aromatic amine
- Aryl chloride
- Aryl halide
- Organosulfonic acid amide
- Benzenoid
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Sulfonyl
- Aminosulfonyl compound
- Secondary amine
- Azacycle
- Dialkylthioether
- Thioether
- Sulfenyl compound
- Organic oxide
- Alkyl fluoride
- Organopnictogen compound
- Organosulfur compound
- Organonitrogen compound
- Organofluoride
- Organochloride
- Organohalogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Alkyl halide
- Amine
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-016v-6598300000-da70dfe63bf7c73c6097 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0204900000-34f0fad758381f4a2cad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-3972100000-ee62da0c273fb1a1e4ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-6696000000-705b8d8ea7ed936c7d46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1219000000-a07dd5653590483d3ec3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0200-9727400000-99669726704eeaf7beaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9110000000-a6fb7cf19b5d9fa5d38b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0000900000-c181b59a993d352abac7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0100900000-3355b1287398915326ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dm-2489500000-679f9280b2ccdb12f774 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-c0dbbe24eddc3668a57d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-3004900000-48d5ae0871023e96f0c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9132000000-2d2fb582e563968b7395 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01324 |
|---|
| HMDB ID | HMDB0015419 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Polythiazide |
|---|
| Chemspider ID | 4704 |
|---|
| ChEBI ID | 553832 |
|---|
| PubChem Compound ID | 4870 |
|---|
| Kegg Compound ID | C07766 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|