| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:55:26 UTC |
|---|
| Update Date | 2016-11-09 01:15:31 UTC |
|---|
| Accession Number | CHEM017089 |
|---|
| Identification |
|---|
| Common Name | Fluorescein sodium |
|---|
| Class | Small Molecule |
|---|
| Description | Fluorescein is a manufactured organic compound and dye. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications.
Fluorescein is a fluorophore commonly used in microscopy, in a type of dye laser as the gain medium, in forensics and serology to detect latent blood stains, and in dye tracing. Fluorescein has an absorption maximum at 494 nm and emission maximum of 512 nm (in water). The major derivatives are fluorescein isothiocyanate (FITC) and, in oligonucleotide synthesis, 6-FAM phosphoramidite.
The color of its aqueous solution varies from green to orange as a function of the way it is observed: by reflection or by transmission, as can be noticed in bubble levels, for example, in which fluorescein is added as a colorant to the alcohol filling the tube in order to increase the visibility of the air bubble contained within (thus enhancing the precision of the instrument). More concentrated solutions of fluorescein can even appear red.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
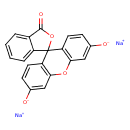
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Fluorescein | MeSH | | Diofluor | MeSH | | Funduscein | MeSH | | Ful glo | MeSH | | Fluorescite | MeSH | | Disodium fluorescein | MeSH | | Fluoresceine, minims | MeSH | | Sodium, fluorescein | MeSH | | D And C yellow no. 7 | MeSH | | Fluorescein disodium salt | MeSH | | Fluor I strip a.t. | MeSH | | Monosodium salt, fluorescein | MeSH | | D And C yellow no. 8 | MeSH | | Fluorescein, disodium | MeSH | | Fluoresceina, colircusi | MeSH | | Sodium fluorescein | MeSH | | Fluorescein sodium | MeSH | | Minims fluoresceine | MeSH | | Fluorescéine sodique faure | MeSH | | Ful-glo | MeSH | | Minims fluorescein sodium | MeSH | | Fluorescein sodium, minims | MeSH | | Optifluor diba | MeSH | | Fluorescein, sodium | MeSH | | Fluorets | MeSH | | Fluorescein monosodium salt | MeSH | | Disodium salt, fluorescein | MeSH | | Fluorescein dipotassium salt | MeSH | | Colircusi fluoresceina | MeSH | | Fluor-I-strip a.t. | MeSH | | Minims stains | MeSH | | Dipotassium salt, fluorescein | MeSH | | Uranine | MeSH | | Disodium 3-oxo-3H-spiro[2-benzofuran-1,9'-xanthene]-3',6'-bis(olic acid) | Generator |
|
|---|
| Chemical Formula | C20H10Na2O5 |
|---|
| Average Molecular Mass | 376.270 g/mol |
|---|
| Monoisotopic Mass | 376.032 g/mol |
|---|
| CAS Registry Number | 518-47-8 |
|---|
| IUPAC Name | disodium 3-oxo-3H-spiro[2-benzofuran-1,9'-xanthene]-3',6'-bis(olate) |
|---|
| Traditional Name | disodium 3-oxospiro[2-benzofuran-1,9'-xanthene]-3',6'-bis(olate) |
|---|
| SMILES | [Na+].[Na+].[O-]C1=CC2=C(C=C1)C1(OC(=O)C3=CC=CC=C13)C1=C(O2)C=C([O-])C=C1 |
|---|
| InChI Identifier | InChI=1S/C20H12O5.2Na/c21-11-5-7-15-17(9-11)24-18-10-12(22)6-8-16(18)20(15)14-4-2-1-3-13(14)19(23)25-20;;/h1-10,21-22H;;/q;2*+1/p-2 |
|---|
| InChI Key | RGPLGPBQJOQFJS-UHFFFAOYSA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthenes. These are polycyclic aromatic compounds containing a xanthene moiety, which consists of two benzene rings joined to each other by a pyran ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Xanthenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthene
- Diaryl ether
- Benzofuranone
- Isobenzofuranone
- Phthalide
- Isocoumaran
- Isobenzofuran
- Phenoxide
- Benzenoid
- Carboxylic acid ester
- Lactone
- Carboxylic acid derivative
- Oxacycle
- Organic alkali metal salt
- Ether
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Organooxygen compound
- Organic zwitterion
- Organic salt
- Organic sodium salt
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-befb723e4011008a66ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0009000000-f863626554c639f367db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00b9-7009000000-00c25a8351dfd514ea9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-5c251e5b37a39b4dc1a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0009000000-5c251e5b37a39b4dc1a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0009000000-5c251e5b37a39b4dc1a7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT000660 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Fluorescein |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 9885981 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|