| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:48:59 UTC |
|---|
| Update Date | 2016-11-09 01:15:30 UTC |
|---|
| Accession Number | CHEM016950 |
|---|
| Identification |
|---|
| Common Name | Methamidophos |
|---|
| Class | Small Molecule |
|---|
| Description | An organic thiophosphate resulting from the N-deacylation of the proinsecticide acephate. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
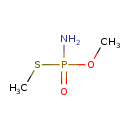
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Metamidophos | ChEBI | | Methyl phosphoramidothioate | ChEBI | | O,S-Dimethyl amidothiophosphate | ChEBI | | O,S-Dimethyl phosphoramidothiolate | ChEBI | | Phosphoramidothioic acid, O,S-dimethyl ester | ChEBI | | Thiophosphoramidic acid O,S-dimethyl ester | ChEBI | | Methyl phosphoramidothioic acid | Generator | | O,S-Dimethyl amidothiophosphoric acid | Generator | | O,S-Dimethyl phosphoramidothiolic acid | Generator | | Phosphoramidothioate, O,S-dimethyl ester | Generator | | Thiophosphoramidate O,S-dimethyl ester | Generator | | Acephate-met | HMDB | | Amidor | HMDB | | Chevron 9006 | HMDB | | Chevron ortho 9006 | HMDB | | Filitox | HMDB | | Hamidop | HMDB | | Metamidofos estrella | HMDB | | Methamidophos | HMDB | | Methyl phosphoramidothioate ((meo)(mes)p(O)(NH2)) | HMDB | | Monitor | HMDB | | Monitor (insecticide) | HMDB | | MTD | HMDB | | O,S-Dimethyl phosphoramidothioate | HMDB | | O,S-Dimethyl phosphoroamidothioate | HMDB | | O,S-Dimethylphosphoroamidothioate | HMDB | | Ortho monitor | HMDB | | Patrole | HMDB | | Pillaron | HMDB | | Sniper | HMDB | | Tahmabon | HMDB | | Tamanox | HMDB | | Tamaron | HMDB |
|
|---|
| Chemical Formula | C2H8NO2PS |
|---|
| Average Molecular Mass | 141.129 g/mol |
|---|
| Monoisotopic Mass | 141.001 g/mol |
|---|
| CAS Registry Number | 10265-92-6 |
|---|
| IUPAC Name | [methoxy(methylsulfanyl)phosphoryl]amine |
|---|
| Traditional Name | tamaron |
|---|
| SMILES | COP(N)(=O)SC |
|---|
| InChI Identifier | InChI=1S/C2H8NO2PS/c1-5-6(3,4)7-2/h1-2H3,(H2,3,4) |
|---|
| InChI Key | NNKVPIKMPCQWCG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphoramidothioic-acid-o,s-diesters. These are organooxygen compounds containing a O,S-diester derivative of phosphoroamidothioic acid. They have the general structure RSP(=O)(OR')(NH2), where R,R' are organyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organophosphorus compounds |
|---|
| Class | Organothiophosphorus compounds |
|---|
| Sub Class | Phosphoramidothioic-acid-O,S-diesters |
|---|
| Direct Parent | Phosphoramidothioic-acid-O,S-diesters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phosphoramidothioic-acid-o,s-diester
- Sulfenyl compound
- Organic nitrogen compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08i4-9300000000-56d4e3bc363cce753683 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0007-7900000000-32e474674de8c15ed60a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0005-9300000000-b1c907e2fd5c6190ef90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-9000000000-19efb41eb42da7683de1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-9700000000-bc4a47915aa2f2d4402f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9000000000-d2352d752bc864cb342c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-9500000000-66b182cec233c6816900 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01ox-9000000000-ac78ce24adc5437da9cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01tc-9000000000-e3aebff85244210b523d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06r2-9200000000-c0c6e8d9e2352da18aa8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-6900000000-f28bfe26553972983789 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9600000000-551cd5d207c6e8ea8c71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dj-9000000000-48066750a628bea43a42 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0031803 |
|---|
| FooDB ID | FDB008476 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 3954 |
|---|
| ChEBI ID | 38721 |
|---|
| PubChem Compound ID | 4096 |
|---|
| Kegg Compound ID | C18667 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|