| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:47:01 UTC |
|---|
| Update Date | 2016-11-09 01:15:29 UTC |
|---|
| Accession Number | CHEM016900 |
|---|
| Identification |
|---|
| Common Name | Testolactone |
|---|
| Class | Small Molecule |
|---|
| Description | An antineoplastic agent that is a derivative of progesterone and used to treat advanced breast cancer. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
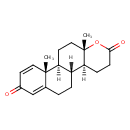
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4AS,4BR,10ar,10BS,12as)-10a,12a-dimethyl-3,4,4a,5,6,10a,10b,11,12,12a-decahydro-2H-naphtho[2,1-F]chromene-2,8(4BH)-dione | ChEBI | | 1,2-Didehydrotestololactone | ChEBI | | 1-Dehydrotestololactone | ChEBI | | 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid delta-lactone | ChEBI | | D-homo-17a-Oxaandrosta-1,4-diene-3,17-dione | ChEBI | | delta(1)-Testololactone | ChEBI | | Teslac | ChEBI | | 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oate delta-lactone | Generator | | 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oate Δ-lactone | Generator | | 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid Δ-lactone | Generator | | Δ(1)-testololactone | Generator | | 1-Dehydrotestolactone | MeSH, HMDB | | 1 Dehydrotestolactone | MeSH, HMDB | | delta(1)-Testolactone | MeSH, HMDB | | 1,2-Dehydrotestololactone | HMDB | | Testolactone | HMDB | | delta1-Dehydrotestololactone | HMDB | | delta1-Testolactone | HMDB | | delta1-Testololactone | HMDB | | Δ1-Dehydrotestololactone | HMDB | | Δ1-Testolactone | HMDB | | Δ1-Testololactone | HMDB |
|
|---|
| Chemical Formula | C19H24O3 |
|---|
| Average Molecular Mass | 300.392 g/mol |
|---|
| Monoisotopic Mass | 300.173 g/mol |
|---|
| CAS Registry Number | 968-93-4 |
|---|
| IUPAC Name | (1R,2S,7S,10S,11R)-7,11-dimethyl-6-oxatetracyclo[8.8.0.0^{2,7}.0^{11,16}]octadeca-12,15-diene-5,14-dione |
|---|
| Traditional Name | (1R,2S,7S,10S,11R)-7,11-dimethyl-6-oxatetracyclo[8.8.0.0^{2,7}.0^{11,16}]octadeca-12,15-diene-5,14-dione |
|---|
| SMILES | [H][C@@]12CCC3=CC(=O)C=C[C@]3(C)[C@@]1([H])CC[C@]1(C)OC(=O)CC[C@@]21[H] |
|---|
| InChI Identifier | InChI=1S/C19H24O3/c1-18-9-7-13(20)11-12(18)3-4-14-15(18)8-10-19(2)16(14)5-6-17(21)22-19/h7,9,11,14-16H,3-6,8,10H2,1-2H3/t14-,15+,16+,18+,19+/m1/s1 |
|---|
| InChI Key | BPEWUONYVDABNZ-DZBHQSCQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthopyrans. Naphthopyrans are compounds containing a pyran ring fused to a naphthalene moiety. Furan is a 6 membered-ring non-aromatic ring with five carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthopyran
- Naphthalene
- Delta valerolactone
- Delta_valerolactone
- Pyran
- Oxane
- Cyclic ketone
- Lactone
- Ketone
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Oxacycle
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05g0-0690000000-49e361148d5394e26c86 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0096000000-cdcc5f9f8e7f59492fe0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pb9-0291000000-34b2744abd958e22e648 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fdo-4590000000-e7db8fb6ab17e0d60421 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052b-0090000000-8efa821a1c48e1557a70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-0090000000-0995c390179d691601d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-7090000000-a32554f7118b988d790a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0029000000-4635acc995962c6e08b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0znc-0892000000-2b63265434d11c289904 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-2900000000-5c09785873ed37a7d2c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-dd82c6133109d0e6e28e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-46bc79dbf2f9c8a57658 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001j-0390000000-357d4c19f4162151e216 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00894 |
|---|
| HMDB ID | HMDB0015031 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Testolactone |
|---|
| Chemspider ID | 13172 |
|---|
| ChEBI ID | 9460 |
|---|
| PubChem Compound ID | 13769 |
|---|
| Kegg Compound ID | C02197 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|