| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:46:18 UTC |
|---|
| Update Date | 2016-11-09 01:15:29 UTC |
|---|
| Accession Number | CHEM016881 |
|---|
| Identification |
|---|
| Common Name | Sulfacytine |
|---|
| Class | Small Molecule |
|---|
| Description | Sulfacytine is a short-acting sulfonamide. The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections.
Sulfacytine is a competitive inhibitor of the enzyme dihydropteroate synthetase. It inhibits bacterial synthesis of of dihydrofolic acid by preventing the condensation of the pteridine with para-aminobenzoic acid (PABA), a substrate of the enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
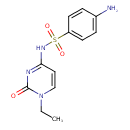
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Renoquid | Kegg | | Sulphacytine | Generator | | 1-Ethyl N4-sulfanilylcytosin | HMDB | | 1-Ethyl-N-sulfanilylcytosine | HMDB | | N-Sulfanilyl-L-ethylcytosine | HMDB | | N(1)-(1-Ethyl-1,2-dihydro-2-oxo-4-pyrimidinyl)sulfanilamide | HMDB |
|
|---|
| Chemical Formula | C12H14N4O3S |
|---|
| Average Molecular Mass | 294.330 g/mol |
|---|
| Monoisotopic Mass | 294.079 g/mol |
|---|
| CAS Registry Number | 17784-12-2 |
|---|
| IUPAC Name | 4-amino-N-(1-ethyl-2-oxo-1,2-dihydropyrimidin-4-yl)benzene-1-sulfonamide |
|---|
| Traditional Name | sulfacytine |
|---|
| SMILES | CCN1C=CC(NS(=O)(=O)C2=CC=C(N)C=C2)=NC1=O |
|---|
| InChI Identifier | InChI=1S/C12H14N4O3S/c1-2-16-8-7-11(14-12(16)17)15-20(18,19)10-5-3-9(13)4-6-10/h3-8H,2,13H2,1H3,(H,14,15,17) |
|---|
| InChI Key | SIBQAECNSSQUOD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aminobenzenesulfonamides. These are organic compounds containing a benzenesulfonamide moiety with an amine group attached to the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonamides |
|---|
| Direct Parent | Aminobenzenesulfonamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzenesulfonamide
- Benzenesulfonyl group
- Aniline or substituted anilines
- Pyrimidone
- Hydropyrimidine
- Pyrimidine
- Organosulfonic acid amide
- Imidolactam
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Sulfonyl
- Aminosulfonyl compound
- Heteroaromatic compound
- Organoheterocyclic compound
- Azacycle
- Hydrocarbon derivative
- Organic nitrogen compound
- Amine
- Organic oxide
- Organopnictogen compound
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organic oxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0699-5970000000-fcf781e9c8d92fac41fe | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0490000000-f5ef4a3fc969278d52f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-1930000000-cca50de9df755740762c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9100000000-0e0f88d63239781bb3dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0190000000-5678559deb4df7cd70e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zml-1590000000-f8b5e6818df98f145ac8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05bf-9710000000-fe4bdffade8dc1239d0f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052b-0790000000-858e661c23fea2a281ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0005-1950000000-8efb821459dc738d0bc6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9310000000-af522fa67546c379ca39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0290000000-14fefa164bb613784c58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dm-0920000000-252f3b6dbf3bb165dace | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-6900000000-bc74a22245fbb377bce2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01298 |
|---|
| HMDB ID | HMDB0015412 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Sulfacytine |
|---|
| Chemspider ID | 5131 |
|---|
| ChEBI ID | 775007 |
|---|
| PubChem Compound ID | 5322 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|