| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:45:52 UTC |
|---|
| Update Date | 2016-11-09 01:15:29 UTC |
|---|
| Accession Number | CHEM016866 |
|---|
| Identification |
|---|
| Common Name | Rofecoxib |
|---|
| Class | Small Molecule |
|---|
| Description | Rofecoxib is used for the treatment of osteoarthritis, rheumatoid arthritis, acute pain in adults, and primary dysmenorrhea, as well as acute treatment of migraine attacks with or without auras. Rofecoxib is a solid. This compound belongs to the stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. Rofecoxib has a half-life of 17 hours and its mean oral bioavailability at therapeutically recommended doses of 125, 25, and 50 mg is approximately 93%. The proteins that rofecoxib target include elastin and prostaglandin G/H synthase 2. Cytochrome P450 1A2, Cytochrome P450 3A4, Cytochrome P450 2C9, Cytochrome P450 2C8, and Prostaglandin G/H synthase 1 are known to metabolize rofecoxib. On September 30, 2004, Merck voluntarily withdrew rofecoxib from the market because of concerns about increased risk of heart attack and stroke associated with long-term, high-dosage use. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
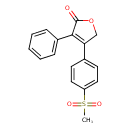
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Phenyl-4-[4-(methylsulfonyl)phenyl]-2(5H)-furanone | ChEBI | | 4-[4-(Methylsulfonyl)phenyl]-3-phenyl-2(5H)-furanone | ChEBI | | Ceoxx | ChEBI | | Rofecoxibum | ChEBI | | Vioxx | ChEBI | | 3-Phenyl-4-[4-(methylsulphonyl)phenyl]-2(5H)-furanone | Generator | | 4-[4-(Methylsulphonyl)phenyl]-3-phenyl-2(5H)-furanone | Generator | | Merck frosst brand OF rofecoxib | MeSH | | Merck sharp and dhome brand OF rofecoxib | MeSH | | Refecoxib | MeSH | | Merck brand OF rofecoxib | MeSH | | Cahill may roberts brand OF rofecoxib | MeSH | | MSD Brand OF rofecoxib | MeSH | | Vioxx dolor | MeSH |
|
|---|
| Chemical Formula | C17H14O4S |
|---|
| Average Molecular Mass | 314.356 g/mol |
|---|
| Monoisotopic Mass | 314.061 g/mol |
|---|
| CAS Registry Number | 162011-90-7 |
|---|
| IUPAC Name | 4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofuran-2-one |
|---|
| Traditional Name | rofecoxib |
|---|
| SMILES | CS(=O)(=O)C1=CC=C(C=C1)C1=C(C(=O)OC1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 |
|---|
| InChI Key | RZJQGNCSTQAWON-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- Benzenesulfonyl group
- Monocyclic benzene moiety
- 2-furanone
- Benzenoid
- Dihydrofuran
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Sulfonyl
- Sulfone
- Carboxylic acid ester
- Lactone
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Organosulfur compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0601-0390000000-d4d8a8654a5376343cab | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-014i-0497000000-44320e93d610fb5074f5 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-014i-0496000000-06716b4dad8f8d33dee6 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0006-2920000000-abe97627fbae89daa0db | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-014i-0497000000-44320e93d610fb5074f5 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-0300900000-8c21a5272c47622769e7 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0002-0390000000-b69da0c82250f1f81243 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-014i-0496000000-06716b4dad8f8d33dee6 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-2920000000-abe97627fbae89daa0db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1129000000-9b4f4aec83cb3607f04c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0400-9288000000-c849d26e51ffb578eff6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9320000000-204a39f3b3ba2ec76d4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0019000000-4ef39ddbe094b85ea63e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-1798000000-e6d03de8c00b576e795c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-5950000000-4dfc8f0e1edbfb7b74dd | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00533 |
|---|
| HMDB ID | HMDB0061179 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Rofecoxib |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 8887 |
|---|
| PubChem Compound ID | 5090 |
|---|
| Kegg Compound ID | C07590 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|