| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:45:51 UTC |
|---|
| Update Date | 2016-11-09 01:15:29 UTC |
|---|
| Accession Number | CHEM016865 |
|---|
| Identification |
|---|
| Common Name | Rocuronium bromide |
|---|
| Class | Small Molecule |
|---|
| Description | The organic bromide salt of a 5alpha androstane compound having 3alpha-hydroxy-, 17beta-acetoxy-, 2beta-morpholino- and 16beta-N-allyllyrrolidinium substituents. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
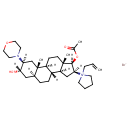
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Allyl-1-(3alpha,17beta-dihydroxy-2beta-morpholino-5alpha-androstan-16beta-yl)pyrrolidinium bromide, 17-acetate | ChEBI | | 17beta-Acetoxy-16beta-(1-allylpyrrolidinium-1-yl)-3alpha-hydroxy-2beta-(morpholin-4-yl)-5alphaandrostane | ChEBI | | Zemuron | Kegg | | 1-Allyl-1-(3a,17b-dihydroxy-2b-morpholino-5a-androstan-16b-yl)pyrrolidinium bromide, 17-acetate | Generator | | 1-Allyl-1-(3a,17b-dihydroxy-2b-morpholino-5a-androstan-16b-yl)pyrrolidinium bromide, 17-acetic acid | Generator | | 1-Allyl-1-(3alpha,17beta-dihydroxy-2beta-morpholino-5alpha-androstan-16beta-yl)pyrrolidinium bromide, 17-acetic acid | Generator | | 1-Allyl-1-(3α,17β-dihydroxy-2β-morpholino-5α-androstan-16β-yl)pyrrolidinium bromide, 17-acetate | Generator | | 1-Allyl-1-(3α,17β-dihydroxy-2β-morpholino-5α-androstan-16β-yl)pyrrolidinium bromide, 17-acetic acid | Generator | | 17b-Acetoxy-16b-(1-allylpyrrolidinium-1-yl)-3a-hydroxy-2b-(morpholin-4-yl)-5alphaandrostane | Generator | | 17Β-acetoxy-16β-(1-allylpyrrolidinium-1-yl)-3α-hydroxy-2β-(morpholin-4-yl)-5alphaandrostane | Generator | | Rocuronium | MeSH | | Pyrrolidinium, 1-((2beta,3alpha,5alpha,16beta,17beta)-17-(acetyloxy)-3-hydroxy-2-(4-morpholinyl)androstan-16-yl)-1-(2-propenyl)-, bromide | MeSH | | Esmerone | MeSH | | 1-(17-(Acetoyl)-3-hydroxy-2-(4-morpholinyl)androstan-16-yl)-1-(2-propenyl)pyrrolidinium | MeSH | | Esmeron | MeSH |
|
|---|
| Chemical Formula | C32H53BrN2O4 |
|---|
| Average Molecular Mass | 609.678 g/mol |
|---|
| Monoisotopic Mass | 608.319 g/mol |
|---|
| CAS Registry Number | 119302-91-9 |
|---|
| IUPAC Name | 1-[(1S,2S,4S,5S,7S,10R,11S,13S,14R,15S)-14-(acetyloxy)-5-hydroxy-2,15-dimethyl-4-(morpholin-4-yl)tetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-13-yl]-1-(prop-2-en-1-yl)pyrrolidin-1-ium bromide |
|---|
| Traditional Name | rocuronium bromide |
|---|
| SMILES | [Br-].[H][C@@]12C[C@@]([H])([C@]([H])(OC(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C[C@]([H])(O)[C@]([H])(C[C@]12C)N1CCOCC1)[N+]1(CC=C)CCCC1 |
|---|
| InChI Identifier | InChI=1S/C32H53N2O4.BrH/c1-5-14-34(15-6-7-16-34)28-20-26-24-9-8-23-19-29(36)27(33-12-17-37-18-13-33)21-32(23,4)25(24)10-11-31(26,3)30(28)38-22(2)35;/h5,23-30,36H,1,6-21H2,2-4H3;1H/q+1;/p-1/t23-,24+,25-,26-,27-,28-,29-,30-,31-,32-;/m0./s1 |
|---|
| InChI Key | OYTJKRAYGYRUJK-FMCCZJBLSA-M |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid esters. Steroid esters are compounds containing a steroid moiety which bears a carboxylic acid ester group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid esters |
|---|
| Direct Parent | Steroid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid ester
- Androgen-skeleton
- Androstane-skeleton
- 3-hydroxysteroid
- Hydroxysteroid
- 3-alpha-hydroxysteroid
- Morpholine
- Oxazinane
- N-alkylpyrrolidine
- Cyclic alcohol
- Tetraalkylammonium salt
- Pyrrolidine
- Quaternary ammonium salt
- 1,2-aminoalcohol
- Amino acid or derivatives
- Carboxylic acid ester
- Secondary alcohol
- Tertiary amine
- Tertiary aliphatic amine
- Organoheterocyclic compound
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Azacycle
- Organic salt
- Amine
- Organic oxide
- Alcohol
- Organic nitrogen compound
- Carbonyl group
- Organopnictogen compound
- Hydrocarbon derivative
- Organic zwitterion
- Organonitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Organic bromide salt
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03xu-0000391000-8b86af482a541e65972c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0i0v-1201690000-ffc41276a30bdf79dafc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03y4-4706930000-fec7650d0c8849e04987 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-2300098000-b1d3dc0659d3237e1939 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kk-1400091000-34ea240a11e1b93873fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-5801970000-e4b826131b384662a2b8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT000575 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Rocuronium_bromide |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 8885 |
|---|
| PubChem Compound ID | 441351 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|