| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:45:43 UTC |
|---|
| Update Date | 2016-11-09 01:15:29 UTC |
|---|
| Accession Number | CHEM016858 |
|---|
| Identification |
|---|
| Common Name | Quinethazone |
|---|
| Class | Small Molecule |
|---|
| Description | Quinethazone, marketed as Hydromox, is a thiazide diuretic indicated for hypertension. Patients may experience adverse reactions such as dizziness, dry mouth, nausea, and hypokalemia. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
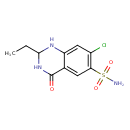
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7-Chloro-2-ethyl-1,2,3,4-tetrahydro-4-oxo-6-quinazolinesulfonamide | ChEBI | | Hydromox | Kegg | | 7-Chloro-2-ethyl-1,2,3,4-tetrahydro-4-oxo-6-quinazolinesulphonamide | Generator | | Chinetazone | HMDB | | Chinethazonum | HMDB | | Quinethazon | HMDB | | Quinethazone, (-)-isomer | HMDB | | Quinethazone, (+)-isomer | HMDB | | Quinethazone, (+-)-isomer | HMDB |
|
|---|
| Chemical Formula | C10H12ClN3O3S |
|---|
| Average Molecular Mass | 289.739 g/mol |
|---|
| Monoisotopic Mass | 289.029 g/mol |
|---|
| CAS Registry Number | 73-49-4 |
|---|
| IUPAC Name | 7-chloro-2-ethyl-4-oxo-1,2,3,4-tetrahydroquinazoline-6-sulfonamide |
|---|
| Traditional Name | quinethazone |
|---|
| SMILES | CCC1NC(=O)C2=CC(=C(Cl)C=C2N1)S(N)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C10H12ClN3O3S/c1-2-9-13-7-4-6(11)8(18(12,16)17)3-5(7)10(15)14-9/h3-4,9,13H,2H2,1H3,(H,14,15)(H2,12,16,17) |
|---|
| InChI Key | AGMMTXLNIQSRCG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as quinazolines. Quinazolines are compounds containing a quinazoline moiety, which is made up of two fused six-member aromatic rings, a benzene ring and a pyrimidine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazanaphthalenes |
|---|
| Sub Class | Benzodiazines |
|---|
| Direct Parent | Quinazolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Quinazoline
- Secondary aliphatic/aromatic amine
- Aryl chloride
- Aryl halide
- Benzenoid
- Organosulfonic acid amide
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Vinylogous amide
- Aminosulfonyl compound
- Sulfonyl
- Amino acid or derivatives
- Carboxamide group
- Lactam
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Secondary amine
- Azacycle
- Organic oxide
- Organopnictogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Amine
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-2390000000-828ade4d50b30315d546 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-01ox-1920000000-83ac1cb82abae8f21317 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-01ox-1920000000-83ac1cb82abae8f21317 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-819817f5299ed4a5a5a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0190000000-9138a4434d98f1a3deac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kbf-2930000000-51b4c473f0cc943f8e17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-cf5cacd5585af23c7d98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002o-9030000000-f28a258ec9dcb0de88ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-2a66bcada53964eb7a81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-17e11cf047e40868380e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-ed1a8b3aadacabbbf440 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0790000000-9af5ce63b502272e04bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-e0705486245b4c3a1765 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-2090000000-bef3e96ae135368141a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-9300000000-5d3c830489a4dffde745 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01325 |
|---|
| HMDB ID | HMDB0015420 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Quinethazone |
|---|
| Chemspider ID | 6068 |
|---|
| ChEBI ID | 8717 |
|---|
| PubChem Compound ID | 6307 |
|---|
| Kegg Compound ID | C07342 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|