| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:45:23 UTC |
|---|
| Update Date | 2016-11-09 01:15:29 UTC |
|---|
| Accession Number | CHEM016848 |
|---|
| Identification |
|---|
| Common Name | Proglumide |
|---|
| Class | Small Molecule |
|---|
| Description | A racemate composed of equal amounts of (R)- and (S)-proglumide. A non-selective CCK antagonist that was used primarily for treatment of stomach ulcers, but has been replaced by newer drugs. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
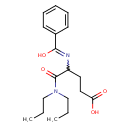
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+-)-4-Benzamido-N,N-dipropylglutaramic acid | ChEBI | | (+-)-Proglumide | ChEBI | | (RS)-Proglumide | ChEBI | | DL-Proglumide | ChEBI | | Proglumida | ChEBI | | Proglumidum | ChEBI | | Racemic proglumide | ChEBI | | Nulsa | Kegg | | (+-)-4-Benzamido-N,N-dipropylglutaramate | Generator | | 4-benzamido-5-(dipropylamino)-5-Oxopentanoate | Generator | | Xylamide | MeSH | | Milid | MeSH | | Proglumide | MeSH | | Xilamide | MeSH |
|
|---|
| Chemical Formula | C18H26N2O4 |
|---|
| Average Molecular Mass | 334.416 g/mol |
|---|
| Monoisotopic Mass | 334.189 g/mol |
|---|
| CAS Registry Number | 6620-60-6 |
|---|
| IUPAC Name | 4-(dipropylcarbamoyl)-4-{[hydroxy(phenyl)methylidene]amino}butanoic acid |
|---|
| Traditional Name | milide |
|---|
| SMILES | CCCN(CCC)C(=O)C(CCC(O)=O)N=C(O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C18H26N2O4/c1-3-12-20(13-4-2)18(24)15(10-11-16(21)22)19-17(23)14-8-6-5-7-9-14/h5-9,15H,3-4,10-13H2,1-2H3,(H,19,23)(H,21,22) |
|---|
| InChI Key | DGMKFQYCZXERLX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Glutamic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glutamic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- Hippuric acid or derivatives
- Alpha-amino acid amide
- Benzamide
- Benzoic acid or derivatives
- Benzoyl
- Monocyclic benzene moiety
- Benzenoid
- N-acyl-amine
- Tertiary carboxylic acid amide
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-5391000000-f2cca4be43844953da18 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0a4i-5921100000-c874128dd39584e6e80b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0a4i-5921100000-c874128dd39584e6e80b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-1359000000-e461c6506cb3050522f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-3941000000-9961f623b953ec670485 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9500000000-7cdf6de929d78ec28e88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0139000000-ba6ab6da2ed84b8a7312 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-3893000000-5da8eeca7fa234faccfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0adl-9730000000-d5405c9f0203b65f1b61 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13431 |
|---|
| HMDB ID | HMDB0256796 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Proglumide |
|---|
| Chemspider ID | 4753 |
|---|
| ChEBI ID | 32058 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|