| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:44:57 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016831 |
|---|
| Identification |
|---|
| Common Name | Phthalylsulfathiazole |
|---|
| Class | Small Molecule |
|---|
| Description | A sulfonamide incorporating 2-carboxybenzamido and 1,3-thiazol-2-yl moieties that is a broad-spectrum antibiotic indicated in the treatment of dysentery, colitis, gastroenteritis and intestinal surgery. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
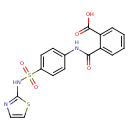
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ftalilsulfatiazol | ChEBI | | Phtalylsulfathiazol | ChEBI | | Phthalylnorsulfazole | ChEBI | | Phthalylsulfathiazolum | ChEBI | | Phthalylsulfonazole | ChEBI | | Phthalylsulphathiazole | ChEBI | | Sulfaphthalazole | ChEBI | | Sulfathalidine | ChEBI | | Sulphaphthalyl | ChEBI | | 2-[[[4-[(2-Thiazolylamino)sulfonyl]phenyl]amino]carbonyl]benzoic acid | Kegg | | Ftalilsulphatiazol | Generator | | Phtalylsulphathiazol | Generator | | Phthalylnorsulphazole | Generator | | Phthalylsulphathiazolum | Generator | | Phthalylsulphonazole | Generator | | Sulphaphthalazole | Generator | | Sulphathalidine | Generator | | Sulfaphthalyl | Generator | | 2-[[[4-[(2-Thiazolylamino)sulfonyl]phenyl]amino]carbonyl]benzoate | Generator | | 2-[[[4-[(2-Thiazolylamino)sulphonyl]phenyl]amino]carbonyl]benzoate | Generator | | 2-[[[4-[(2-Thiazolylamino)sulphonyl]phenyl]amino]carbonyl]benzoic acid | Generator | | Phthalazol | MeSH | | Ftalazol | MeSH | | Phthalylsulfathiazole monosodium salt | MeSH | | Phthalylsulfathiazole | KEGG | | 2-({4-[(1,3-thiazol-2-yl)sulfamoyl]phenyl}carbamoyl)benzoate | Generator | | 2-({4-[(1,3-thiazol-2-yl)sulphamoyl]phenyl}carbamoyl)benzoate | Generator | | 2-({4-[(1,3-thiazol-2-yl)sulphamoyl]phenyl}carbamoyl)benzoic acid | Generator |
|
|---|
| Chemical Formula | C17H13N3O5S2 |
|---|
| Average Molecular Mass | 403.430 g/mol |
|---|
| Monoisotopic Mass | 403.030 g/mol |
|---|
| CAS Registry Number | 85-73-4 |
|---|
| IUPAC Name | 2-({4-[(1,3-thiazol-2-yl)sulfamoyl]phenyl}carbamoyl)benzoic acid |
|---|
| Traditional Name | phthalylsulphathiazole |
|---|
| SMILES | OC(=O)C1=CC=CC=C1C(=O)NC1=CC=C(C=C1)S(=O)(=O)NC1=NC=CS1 |
|---|
| InChI Identifier | InChI=1S/C17H13N3O5S2/c21-15(13-3-1-2-4-14(13)16(22)23)19-11-5-7-12(8-6-11)27(24,25)20-17-18-9-10-26-17/h1-10H,(H,18,20)(H,19,21)(H,22,23) |
|---|
| InChI Key | PBMSWVPMRUJMPE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzanilides. These are aromatic compounds containing an anilide group in which the carboxamide group is substituted with a benzene ring. They have the general structure RNC(=O)R', where R,R'= benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Anilides |
|---|
| Direct Parent | Benzanilides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzanilide
- Benzenesulfonamide
- Benzamide
- Benzoic acid or derivatives
- Benzoic acid
- Benzenesulfonyl group
- Benzoyl
- Organosulfonic acid amide
- Azole
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Heteroaromatic compound
- Sulfonyl
- Thiazole
- Aminosulfonyl compound
- Secondary carboxylic acid amide
- Carboxamide group
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organoheterocyclic compound
- Azacycle
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pbj-7944000000-8d3c4a0755194cecee74 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-052b-3940000000-92fc3db27cdfa654a5fd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-052b-3940000000-92fc3db27cdfa654a5fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0004900000-b6ca51ba5e57c559dc9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-0439300000-23f8498a44a355d56de5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fl0-6931000000-37c4b38fb96fd4144580 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0004900000-eff2850819bea30578f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-11ou-2009100000-fc36f3f5ac4eae050438 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-8889000000-42420c5c8401039609b1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13248 |
|---|
| HMDB ID | HMDB0256507 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Phthalylsulfathiazole |
|---|
| Chemspider ID | 4641 |
|---|
| ChEBI ID | 9336 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C07659 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|