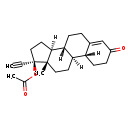
| (17-alpha)-Norethindrone acetate | ChEBI |
| 17-Acetoxy-19-nor-17-alpha-pregn-4-en-20-yn-3-one | ChEBI |
| 17-Acetoxy-19-nor-17alpha-pregn-4-en-20-yn-3-one | ChEBI |
| 17-Acetyloxy(17-alpha)-19-norpregn-4-estren-17-beta-ol-acetate-3-one | ChEBI |
| 17-alpha-Ethinyl-19-nortestosterone acetate | ChEBI |
| 17-alpha-Ethinyl-19-nortestosterone-17-beta-acetate | ChEBI |
| 17-alpha-Ethynyl-17-hydroxyestr-4-en-3-one acetate | ChEBI |
| 17-alpha-Ethynyl-19-nortestosterone acetate | ChEBI |
| 17-Hydroxy-19-nor-17-alpha-pregn-4-en-20-yn-3-one acetate | ChEBI |
| 17-Hydroxy-19-nor-17alpha-pregn-4-en-20-yn-3-one acetate | ChEBI |
| 17alpha-Ethinyl-19-nortestosterone 17beta-acetate | ChEBI |
| 17alpha-Ethinyl-19-nortestosterone-17beta-acetate | ChEBI |
| 17alpha-Ethynyl-17-hydroxyestr-4-en-3-one acetate | ChEBI |
| 17alpha-Ethynyl-17beta-acetoxy-19-norandrost-4-en-3-one | ChEBI |
| 17alpha-Ethynyl-19-nortestosterone acetate | ChEBI |
| 17beta-Acetoxy-19-nor-17alpha-pregn-4-en-20-yn-3-one | ChEBI |
| 17beta-Hydroxy-19-nor-17alpha-pregn-4-en-20-yn-3-one acetate | ChEBI |
| 19-Norethindrone acetate | ChEBI |
| 19-Norethisterone acetate | ChEBI |
| 19-Norethynyltestosterone acetate | ChEBI |
| Aygestin | ChEBI |
| Norethindrone 17-acetate | ChEBI |
| Norethindrone acetate | ChEBI |
| Norlutate | ChEBI |
| (17-a)-Norethindrone acetate | Generator |
| (17-a)-Norethindrone acetic acid | Generator |
| (17-alpha)-Norethindrone acetic acid | Generator |
| (17-Α)-norethindrone acetate | Generator |
| (17-Α)-norethindrone acetic acid | Generator |
| 17-Acetoxy-19-nor-17-a-pregn-4-en-20-yn-3-one | Generator |
| 17-Acetoxy-19-nor-17-α-pregn-4-en-20-yn-3-one | Generator |
| 17-Acetoxy-19-nor-17a-pregn-4-en-20-yn-3-one | Generator |
| 17-Acetoxy-19-nor-17α-pregn-4-en-20-yn-3-one | Generator |
| 17-Acetyloxy(17-a)-19-norpregn-4-estren-17-b-ol-acetate-3-one | Generator |
| 17-Acetyloxy(17-a)-19-norpregn-4-estren-17-b-ol-acetic acid-3-one | Generator |
| 17-Acetyloxy(17-alpha)-19-norpregn-4-estren-17-beta-ol-acetic acid-3-one | Generator |
| 17-Acetyloxy(17-α)-19-norpregn-4-estren-17-β-ol-acetate-3-one | Generator |
| 17-Acetyloxy(17-α)-19-norpregn-4-estren-17-β-ol-acetic acid-3-one | Generator |
| 17-a-Ethinyl-19-nortestosterone acetate | Generator |
| 17-a-Ethinyl-19-nortestosterone acetic acid | Generator |
| 17-alpha-Ethinyl-19-nortestosterone acetic acid | Generator |
| 17-Α-ethinyl-19-nortestosterone acetate | Generator |
| 17-Α-ethinyl-19-nortestosterone acetic acid | Generator |
| 17-a-Ethinyl-19-nortestosterone-17-b-acetate | Generator |
| 17-a-Ethinyl-19-nortestosterone-17-b-acetic acid | Generator |
| 17-alpha-Ethinyl-19-nortestosterone-17-beta-acetic acid | Generator |
| 17-Α-ethinyl-19-nortestosterone-17-β-acetate | Generator |
| 17-Α-ethinyl-19-nortestosterone-17-β-acetic acid | Generator |
| 17-a-Ethynyl-17-hydroxyestr-4-en-3-one acetate | Generator |
| 17-a-Ethynyl-17-hydroxyestr-4-en-3-one acetic acid | Generator |
| 17-alpha-Ethynyl-17-hydroxyestr-4-en-3-one acetic acid | Generator |
| 17-Α-ethynyl-17-hydroxyestr-4-en-3-one acetate | Generator |
| 17-Α-ethynyl-17-hydroxyestr-4-en-3-one acetic acid | Generator |
| 17-a-Ethynyl-19-nortestosterone acetate | Generator |
| 17-a-Ethynyl-19-nortestosterone acetic acid | Generator |
| 17-alpha-Ethynyl-19-nortestosterone acetic acid | Generator |
| 17-Α-ethynyl-19-nortestosterone acetate | Generator |
| 17-Α-ethynyl-19-nortestosterone acetic acid | Generator |
| 17-Hydroxy-19-nor-17-a-pregn-4-en-20-yn-3-one acetate | Generator |
| 17-Hydroxy-19-nor-17-a-pregn-4-en-20-yn-3-one acetic acid | Generator |
| 17-Hydroxy-19-nor-17-alpha-pregn-4-en-20-yn-3-one acetic acid | Generator |
| 17-Hydroxy-19-nor-17-α-pregn-4-en-20-yn-3-one acetate | Generator |
| 17-Hydroxy-19-nor-17-α-pregn-4-en-20-yn-3-one acetic acid | Generator |
| 17-Hydroxy-19-nor-17a-pregn-4-en-20-yn-3-one acetate | Generator |
| 17-Hydroxy-19-nor-17a-pregn-4-en-20-yn-3-one acetic acid | Generator |
| 17-Hydroxy-19-nor-17alpha-pregn-4-en-20-yn-3-one acetic acid | Generator |
| 17-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetate | Generator |
| 17-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetic acid | Generator |
| 17a-Ethinyl-19-nortestosterone 17b-acetate | Generator |
| 17a-Ethinyl-19-nortestosterone 17b-acetic acid | Generator |
| 17alpha-Ethinyl-19-nortestosterone 17beta-acetic acid | Generator |
| 17Α-ethinyl-19-nortestosterone 17β-acetate | Generator |
| 17Α-ethinyl-19-nortestosterone 17β-acetic acid | Generator |
| 17a-Ethinyl-19-nortestosterone-17b-acetate | Generator |
| 17a-Ethinyl-19-nortestosterone-17b-acetic acid | Generator |
| 17alpha-Ethinyl-19-nortestosterone-17beta-acetic acid | Generator |
| 17Α-ethinyl-19-nortestosterone-17β-acetate | Generator |
| 17Α-ethinyl-19-nortestosterone-17β-acetic acid | Generator |
| 17a-Ethynyl-17-hydroxyestr-4-en-3-one acetate | Generator |
| 17a-Ethynyl-17-hydroxyestr-4-en-3-one acetic acid | Generator |
| 17alpha-Ethynyl-17-hydroxyestr-4-en-3-one acetic acid | Generator |
| 17Α-ethynyl-17-hydroxyestr-4-en-3-one acetate | Generator |
| 17Α-ethynyl-17-hydroxyestr-4-en-3-one acetic acid | Generator |
| 17a-Ethynyl-17b-acetoxy-19-norandrost-4-en-3-one | Generator |
| 17Α-ethynyl-17β-acetoxy-19-norandrost-4-en-3-one | Generator |
| 17a-Ethynyl-19-nortestosterone acetate | Generator |
| 17a-Ethynyl-19-nortestosterone acetic acid | Generator |
| 17alpha-Ethynyl-19-nortestosterone acetic acid | Generator |
| 17Α-ethynyl-19-nortestosterone acetate | Generator |
| 17Α-ethynyl-19-nortestosterone acetic acid | Generator |
| 17b-Acetoxy-19-nor-17a-pregn-4-en-20-yn-3-one | Generator |
| 17Β-acetoxy-19-nor-17α-pregn-4-en-20-yn-3-one | Generator |
| 17b-Hydroxy-19-nor-17a-pregn-4-en-20-yn-3-one acetate | Generator |
| 17b-Hydroxy-19-nor-17a-pregn-4-en-20-yn-3-one acetic acid | Generator |
| 17beta-Hydroxy-19-nor-17alpha-pregn-4-en-20-yn-3-one acetic acid | Generator |
| 17Β-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetate | Generator |
| 17Β-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetic acid | Generator |
| 19-Norethindrone acetic acid | Generator |
| 19-Norethisterone acetic acid | Generator |
| 19-Norethynyltestosterone acetic acid | Generator |
| Norethindrone 17-acetic acid | Generator |
| Norethindrone acetic acid | Generator |
| Norlutic acid | Generator |
| Norethisterone acetic acid | Generator |
| Norethindrone acetate, (17alpha)-isomer | MeSH |
| Norethindrone acetate, (17alpha)-(+-)-isomer | MeSH |