| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:44:24 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016812 |
|---|
| Identification |
|---|
| Common Name | Nomifensine |
|---|
| Class | Small Molecule |
|---|
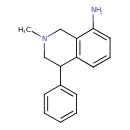
| Description | An N-methylated tetrahydroisoquinoline carrying phenyl and amino substituents at positions C-4 and C-8, respectively. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+-)-Nomifensin | ChEBI | | (+-)-Nomifensine | ChEBI | | 2-Methyl-4-phenyl-1,2,3,4-tetrahydro-isoquinolin-8-ylamine | ChEBI | | 8-Amino-2-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinoline | ChEBI | | D,L-Nomifensine | ChEBI | | Nomifenison | ChEBI | | Nomifensin | ChEBI | | Nomifensina | ChEBI | | Nomifensinum | ChEBI | | Nomiphensine | ChEBI | | R/S-nomifensine | ChEBI | | Hoe 984 | MeSH | | Hoe-984 | MeSH | | Linamiphen | MeSH | | Maleate, nomifensine | MeSH | | Merital | MeSH | | Nomifensine maleate | MeSH | | Nomifensine maleate (1:1) | MeSH |
|
|---|
| Chemical Formula | C16H18N2 |
|---|
| Average Molecular Mass | 238.328 g/mol |
|---|
| Monoisotopic Mass | 238.147 g/mol |
|---|
| CAS Registry Number | 24526-64-5 |
|---|
| IUPAC Name | 2-methyl-4-phenyl-1,2,3,4-tetrahydroisoquinolin-8-amine |
|---|
| Traditional Name | nomifensine |
|---|
| SMILES | CN1CC(C2=CC=CC=C2)C2=C(C1)C(N)=CC=C2 |
|---|
| InChI Identifier | InChI=1S/C16H18N2/c1-18-10-14(12-6-3-2-4-7-12)13-8-5-9-16(17)15(13)11-18/h2-9,14H,10-11,17H2,1H3 |
|---|
| InChI Key | XXPANQJNYNUNES-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 4-phenyltetrahydroisoquinolines. 4-phenyltetrahydroisoquinolines are compounds containing a phenyl group attached to the C4-atom of a tetrahydroisoquinoline moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydroisoquinolines |
|---|
| Sub Class | 4-phenyltetrahydroisoquinolines |
|---|
| Direct Parent | 4-phenyltetrahydroisoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4-phenyltetrahydroisoquinoline
- Aminoquinoline
- Aralkylamine
- Benzenoid
- Monocyclic benzene moiety
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organonitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0072-0970000000-ad3c343f6ca7a3cb9820 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-00lm-2940000000-7452f23561e71b6e5f4a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-000i-0290000000-aa9e7f721070bb0efac0 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-000i-0090000000-9b5661cbfd28f31b72a9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-00lm-2940000000-fd95609b0f83c449fc1e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-015c-2900000000-1fc47bf732f7fe98aac4 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-015c-2900000000-3295b45d4791d9153cb4 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-00l6-3900000000-6d615d80f77aabb59983 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0079-0190000000-745862c4f89a54e632cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0077-3980000000-9e714093a641e3d13424 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kc7-1910000000-b630745ed8db1f8a5799 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-e1d4264595b4b490c554 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0190000000-ec66f46afc099fca91fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00fu-6690000000-a62b409ce47dcc5fade0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04821 |
|---|
| HMDB ID | HMDB0255702 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Nomifensine |
|---|
| Chemspider ID | 4371 |
|---|
| ChEBI ID | 116225 |
|---|
| PubChem Compound ID | 4528 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|