| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:44:20 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016808 |
|---|
| Identification |
|---|
| Common Name | Nimodipine |
|---|
| Class | Small Molecule |
|---|
| Description | Nimodipine is a 1,4-dihydropyridine calcium channel blocker. It acts primarily on vascular smooth muscle cells by stabilizing voltage-gated L-type calcium channels in their inactive conformation. By inhibiting the influx of calcium in smooth muscle cells, nimodipine prevents calcium-dependent smooth muscle contraction and subsequent vasoconstriction. Compared to other calcium channel blocking agents, nimodipine exhibits greater effects on cerebral circulation than on peripheral circulation. Nimodipine is used to as an adjunct to improve the neurologic outcome following subarachnoid hemorrhage from ruptured intracranial aneurysm. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
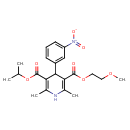
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,6-Dimethyl-4-(3'-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid 3-beta-methoxyethyl ester 5-isopropyl ester | ChEBI | | BAY e 9736 | ChEBI | | Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate | ChEBI | | Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate | ChEBI | | Nimodipino | ChEBI | | Nimodipinum | ChEBI | | Nimotop | ChEBI | | Periplum | ChEBI | | Nymalize | Kegg | | 2,6-Dimethyl-4-(3'-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate 3-b-methoxyethyl ester 5-isopropyl ester | Generator | | 2,6-Dimethyl-4-(3'-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate 3-beta-methoxyethyl ester 5-isopropyl ester | Generator | | 2,6-Dimethyl-4-(3'-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate 3-β-methoxyethyl ester 5-isopropyl ester | Generator | | 2,6-Dimethyl-4-(3'-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid 3-b-methoxyethyl ester 5-isopropyl ester | Generator | | 2,6-Dimethyl-4-(3'-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid 3-β-methoxyethyl ester 5-isopropyl ester | Generator | | Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid | Generator | | Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylic acid | Generator | | Andromaco brand OF nimodipine | HMDB | | Bayvit, nimodipino | HMDB | | Cantabria brand OF nimodipine | HMDB | | Hexal, nimodipin | HMDB | | Alpharma brand OF nimodipine | HMDB | | Bayvit brand OF nimodipine | HMDB | | Kenesil | HMDB | | Admon | HMDB | | Brainal | HMDB | | Modus | HMDB | | Nimodipin hexal | HMDB | | Nimodipin-isis | HMDB | | NimodipinISIS | HMDB | | Nimodipino bayvit | HMDB | | Vita brand OF nimodipine | HMDB | | e 9736, Bay | HMDB | | 9736, Bay e | HMDB | | Almirall brand OF nimodipine | HMDB | | Bayer brand OF nimodipine | HMDB | | Calnit | HMDB | | Elan brand OF nimodipine | HMDB | | Esteve brand OF nimodipine | HMDB | | Hexal brand OF nimodipine | HMDB | | Nimodipin isis | HMDB | | Remontal | HMDB |
|
|---|
| Chemical Formula | C21H26N2O7 |
|---|
| Average Molecular Mass | 418.440 g/mol |
|---|
| Monoisotopic Mass | 418.174 g/mol |
|---|
| CAS Registry Number | 66085-59-4 |
|---|
| IUPAC Name | 3-(2-methoxyethyl) 5-propan-2-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate |
|---|
| Traditional Name | nimodipine |
|---|
| SMILES | COCCOC(=O)C1=C(C)NC(C)=C(C1C1=CC(=CC=C1)[N+]([O-])=O)C(=O)OC(C)C |
|---|
| InChI Identifier | InChI=1S/C21H26N2O7/c1-12(2)30-21(25)18-14(4)22-13(3)17(20(24)29-10-9-28-5)19(18)15-7-6-8-16(11-15)23(26)27/h6-8,11-12,19,22H,9-10H2,1-5H3 |
|---|
| InChI Key | UIAGMCDKSXEBJQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydropyridinecarboxylic acids and derivatives. Dihydropyridinecarboxylic acids and derivatives are compounds containing a dihydropyridine moiety bearing a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Hydropyridines |
|---|
| Direct Parent | Dihydropyridinecarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydropyridinecarboxylic acid derivative
- Nitrobenzene
- Nitroaromatic compound
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Benzenoid
- Vinylogous amide
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Amino acid or derivatives
- Carboxylic acid ester
- C-nitro compound
- Organic nitro compound
- Carboxylic acid derivative
- Dialkyl ether
- Secondary aliphatic amine
- Enamine
- Ether
- Azacycle
- Organic 1,3-dipolar compound
- Organic oxoazanium
- Secondary amine
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Organic oxygen compound
- Carbonyl group
- Amine
- Organopnictogen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic zwitterion
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f6y-8009000000-df297acbb119ead19ae2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0006-0149000000-2a019013c0386ca13362 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0zfu-0039000000-4fc95af4f67b0afb5234 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-01bc-1654900000-b2486a1feba93aef89fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-5000900000-12c2938a62c8cb238e76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0aor-9002600000-b69caecacf04408cf15b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bt9-9000000000-58c041d65b909517cc19 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-3000900000-d333c69b2de3907d9af1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-9000600000-d1954bdf68414fdaddb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-3a47cedd9c89d41d9be5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0004900000-aedc1fdb52380276f236 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-6049500000-b4e1452bef00eb5c76e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9020000000-24deee9cc6a1174c9f27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014l-0009400000-10fb7090b7ccce0b345e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ap3-0079100000-d496424ed4e3b7807f28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kdi-0098000000-cae1334e08a3166f17ba | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00393 |
|---|
| HMDB ID | HMDB0014537 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Nimodipine |
|---|
| Chemspider ID | 4341 |
|---|
| ChEBI ID | 7575 |
|---|
| PubChem Compound ID | 4497 |
|---|
| Kegg Compound ID | C07267 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Boone SC, Chou SN, Kelly DL, Weir BK, Crabbe RA, Lavik PJ, Rosenbloom SB, Dorsey FC, Ingram CR, Mellits DE, Bertsch LA, Boisvert DP, Hundley MB, Johnson RK, Strom JA, Transou CR: Cerebral arterial spasm--a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983 Mar 17;308(11):619-24. | | 2. Belfort MA, Anthony J, Saade GR, Allen JC Jr: A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. N Engl J Med. 2003 Jan 23;348(4):304-11. | | 3. Janjua N, Mayer SA: Cerebral vasospasm after subarachnoid hemorrhage. Curr Opin Crit Care. 2003 Apr;9(2):113-9. | | 4. Vergouwen MD, Vermeulen M, Roos YB: Effect of nimodipine on outcome in patients with traumatic subarachnoid haemorrhage: a systematic review. Lancet Neurol. 2006 Dec;5(12):1029-32. | | 5. Tomassoni D, Lanari A, Silvestrelli G, Traini E, Amenta F: Nimodipine and its use in cerebrovascular disease: evidence from recent preclinical and controlled clinical studies. Clin Exp Hypertens. 2008 Nov;30(8):744-66. doi: 10.1080/10641960802580232. | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=12137606 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=16180362 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=17110283 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=21869451 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=22262041 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=22300914 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=8519001 |
|
|---|