| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:44:01 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016800 |
|---|
| Identification |
|---|
| Common Name | Nandrolone phenpropionate |
|---|
| Class | Small Molecule |
|---|
| Description | C18 steroid with androgenic and anabolic properties. It is generally prepared from alkyl ethers of estradiol to resemble testosterone but less one carbon at the 19 position. It is a schedule III drug in the U.S. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
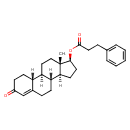
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 19NTPP | ChEBI | | Durabolin | ChEBI | | Nadrolone phenylpropionate | ChEBI | | Nandrolon phenylpropionate | ChEBI | | Nandrolone phenylpionate | ChEBI | | Nandrolone phenylpropionate | ChEBI | | Norandrolone phenyl propionate | ChEBI | | Norandrostenolone phenylpropionate | ChEBI | | Nortestosterone phenylpropionate | ChEBI | | NPP | ChEBI | | NTPP | ChEBI | | Nadrolone phenylpropionic acid | Generator | | Nandrolon phenylpropionic acid | Generator | | Nandrolone phenylpionic acid | Generator | | Nandrolone phenylpropionic acid | Generator | | Norandrolone phenyl propionic acid | Generator | | Norandrostenolone phenylpropionic acid | Generator | | Nortestosterone phenylpropionic acid | Generator | | Nandrolone phenpropionic acid | Generator | | Nandrolin | HMDB | | Testosterone phenylpropionate | HMDB | | 17 beta-Hydroxyestr-4-en-3-one hydrocinnamate | HMDB | | Nandrolone phenpropionate, (17alpha)-isomer | HMDB | | Fenobolin | HMDB | | Nerobolyl | HMDB | | Nerobolil | HMDB | | Norandrolone phenylpropionate | HMDB |
|
|---|
| Chemical Formula | C27H34O3 |
|---|
| Average Molecular Mass | 406.557 g/mol |
|---|
| Monoisotopic Mass | 406.251 g/mol |
|---|
| CAS Registry Number | 62-90-8 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14S,15S)-15-methyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl 3-phenylpropanoate |
|---|
| Traditional Name | (1S,2R,10R,11S,14S,15S)-15-methyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-14-yl 3-phenylpropanoate |
|---|
| SMILES | [H][C@@]12CC[C@H](OC(=O)CCC3=CC=CC=C3)[C@@]1(C)CC[C@]1([H])[C@@]3([H])CCC(=O)C=C3CC[C@@]21[H] |
|---|
| InChI Identifier | InChI=1S/C27H34O3/c1-27-16-15-22-21-11-9-20(28)17-19(21)8-10-23(22)24(27)12-13-25(27)30-26(29)14-7-18-5-3-2-4-6-18/h2-6,17,21-25H,7-16H2,1H3/t21-,22+,23+,24-,25-,27-/m0/s1 |
|---|
| InChI Key | UBWXUGDQUBIEIZ-QNTYDACNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid esters. Steroid esters are compounds containing a steroid moiety which bears a carboxylic acid ester group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid esters |
|---|
| Direct Parent | Steroid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid ester
- Estrogen-skeleton
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- Oxosteroid
- Estrane-skeleton
- Delta-4-steroid
- Cyclohexenone
- Monocyclic benzene moiety
- Benzenoid
- Carboxylic acid ester
- Cyclic ketone
- Ketone
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-056u-2794000000-8a406f2131925f7d78d7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2693800000-014d5a8f5785d5fa2138 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a59-2891000000-0d191fd67047632bac0b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002f-2290000000-d1eb49bea81bc4fe8fec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0250900000-73ea898c756aee0d065d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-0491200000-c48f4cd0cb4d262db924 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-2290000000-2d0ca51aad14a4610e02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2290300000-bac5d57e78f5058c299b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-5931100000-59cd27d9e64a7d2840f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4u-3920000000-ff8ab93696f1c30ae190 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000900000-c12aa2fec47362dfe817 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2311900000-d39466b8e277a338d135 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9707100000-32c75e3ec9135a8c9585 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00984 |
|---|
| HMDB ID | HMDB0015119 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Nandrolone phenylpropionate |
|---|
| Chemspider ID | 199761 |
|---|
| ChEBI ID | 7468 |
|---|
| PubChem Compound ID | 229455 |
|---|
| Kegg Compound ID | C08155 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|