| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 03:44:00 UTC |
|---|
| Update Date | 2016-11-09 01:15:28 UTC |
|---|
| Accession Number | CHEM016799 |
|---|
| Identification |
|---|
| Common Name | Nandrolone decanoate |
|---|
| Class | Small Molecule |
|---|
| Description | C18 steroid with androgenic and anabolic properties. It is generally prepared from alkyl ethers of estradiol to resemble testosterone but less one carbon at the 19 position. It is a schedule III drug in the U.S. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
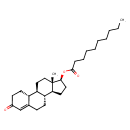
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Deca-durabolin | Kegg | | Nandrolone decanoic acid | Generator | | Norandrostenolone decanoate | HMDB | | Nortestosterone decanoate | HMDB | | 19-Nor-4-androstene-17 beta-ol-3-one 17-decanoate | HMDB | | Decadurobolin | HMDB | | 17 beta-Hydroxyestr-4-en-3-one 17-decanoate | HMDB | | Retabolil | HMDB | | Retabolyl | HMDB | | 19-Nortestosterone decanoate | HMDB | | Decadurabolin | HMDB |
|
|---|
| Chemical Formula | C28H44O3 |
|---|
| Average Molecular Mass | 428.647 g/mol |
|---|
| Monoisotopic Mass | 428.329 g/mol |
|---|
| CAS Registry Number | 360-70-3 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14S,15S)-15-methyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-14-yl decanoate |
|---|
| Traditional Name | nandrolone decanoate |
|---|
| SMILES | CCCCCCCCCC(=O)O[C@H]1CC[C@H]2[C@@H]3CCC4=CC(=O)CC[C@@H]4[C@H]3CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C28H44O3/c1-3-4-5-6-7-8-9-10-27(30)31-26-16-15-25-24-13-11-20-19-21(29)12-14-22(20)23(24)17-18-28(25,26)2/h19,22-26H,3-18H2,1-2H3/t22-,23+,24+,25-,26-,28-/m0/s1 |
|---|
| InChI Key | JKWKMORAXJQQSR-MOPIKTETSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroid esters. Steroid esters are compounds containing a steroid moiety which bears a carboxylic acid ester group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroid esters |
|---|
| Direct Parent | Steroid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroid ester
- Estrogen-skeleton
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- Oxosteroid
- Estrane-skeleton
- Delta-4-steroid
- Cyclohexenone
- Carboxylic acid ester
- Cyclic ketone
- Ketone
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pe9-2492100000-fc574c5790f53b7819cc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0660900000-27b3f9df49a3122ab78d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-2981100000-1a157ce9c28d39dbdaf0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0096-5290000000-aedae95d8afd82e740fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0240900000-e7b5d0ebbd228f9935cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0490300000-83cbc30a58b63f57d9c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-2190000000-739839f2f5e3d2cd72ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0040900000-472c8f65fe7d03ec685f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-6393400000-96dc1d469aa36aa88972 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9310000000-e5bf97875dceb16d7997 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000900000-a509d4af6bebc9e05806 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0210900000-878845e4a683c8e5bba9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-9611300000-d0c86689a7513cb4ea58 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB08804 |
|---|
| HMDB ID | HMDB0015694 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Nandrolone decanoate |
|---|
| Chemspider ID | 9296 |
|---|
| ChEBI ID | 774897 |
|---|
| PubChem Compound ID | 9677 |
|---|
| Kegg Compound ID | C08154 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|